How many times have we seen bands from IgG heavy or light chains interfering with our bands of interest? Typically the source of this problem is the secondary antibody we use. Secondary antibodies are directed against the IgG class or subclass of the species in which the primary antibody was raised. In general, they react with both the IgG heavy and light chain.
Besides the desired binding to the primary antibody, heavy and light chain–specific secondary antibodies tend to bind to heavy and light chains of endogenous immunoglobulin molecules present in reduced or denatured western blot samples. In addition to the endogenous IgGs, primary antibodies of the IgG isotypes are also released from beads during immunoprecipitation (IP) procedures. Bands from IgG heavy (~50 kD) and light (~25 kD) chains often mask or obstruct the bands of interest in a western blot of immunoprecipitated proteins.
How do you avoid visualization of IgG heavy or light chains when your immunoprecipitated protein of interest has a molecular weight close to 25 or 50 kD?
The best way to avoid this problem is to use a secondary antibody or a detection reagent that binds only to the native nondenatured antibodies rather than to any IgG present in the sample. This is a sure way to reduce or eliminate the problem of background band detection in western blots. The new detection reagent from Bio-Rad, TidyBlot™ Western Blot Detection Reagent, does exactly that. It is HRP conjugated and binds exclusively to the native nondenatured IgG antibody used for western blot detection and thereby binds only to the IgG of interest. The result is a cleaner western blot, as the IgG heavy and light chain bands do not show up (see Figures 1, 2, and 3 below).
Here are some fun band detection exercises to challenge your western blot–trained eyes:
1. Do you or do you not see the band at 25 kD representing the GSTP1 protein in lane 5?
The following western blot was generated to detect the 25 kD GSTP1 protein. The experimental procedure described in the figure caption was followed except that the TidyBlot Secondary Detection Reagent was used in lane 2 and an HRP-conjugated anti-mouse IgG (heavy and light chain) secondary antibody was used in lane 5. Can you see the 25 kD GSTP1 band hidden behind the IgG band in lane 5?
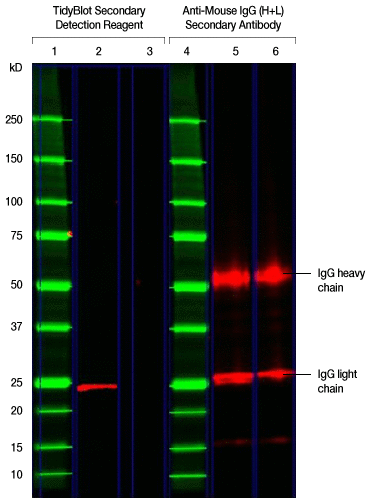
Fig. 1. Detection of the 25 kD GSTP1 protein. TidyBlot Western Blot Detection Reagent:HRP binds exclusively to the native anti-GSTP1 antibody, whereas the HRP-conjugated anti-mouse IgG heavy and light chain secondary antibody recognizes both the native anti-GSTP1 and denatured antibodies (as shown by the detection of heavy and light chains) in this mock IP experiment. K562 cell lysate (25 µg) spiked with 1 µg of mouse monoclonal antibody of the IgG2b isotype was run in lanes 2, 3, 5, and 6 under reduced conditions for SDS-PAGE and the proteins were transferred onto a nitrocellulose membrane. Lanes 1 and 4 were loaded with Precision Plus Protein™ All Blue Protein Standard. Mouse anti-GSTP1 PrecisionAb™ Antibody was used as a primary antibody for the detection of the GSTP1 protein (~25 kD) in lanes 2 and 5. Lanes 3 and 6 were secondary-antibody-only controls (no primary antibody was added) to determine background staining from the secondary detection reagents. TidyBlot Western Blot Detection Reagent:HRP was used as the secondary detection reagent for lanes 2 and 3. An HRP-conjugated anti-mouse IgG (heavy and light chain) secondary antibody was used as the secondary detection reagent for lanes 5 and 6. The blot was visualized with the ChemiDoc™ MP Imaging System. Please note that TidyBlot Reagent is HRP conjugated and that the western blot bands (red) and protein standards (green) have been pseudocolored.
2. Find the band at 50 kD representing the SSB protein in lane 4.
The following western blot was generated to detect the 50 kD SSB (also known as Lupus La) protein. The experimental procedure described in the figure caption was followed except that the TidyBlot Western Blot Detection Reagent was used for the detection of SSB in lane 2 and an HRP-conjugated anti-mouse IgG (heavy and light chain) secondary antibody was used for lane 4. Can you find the 50 kD SSB protein band hidden behind the IgG band in lane 4?
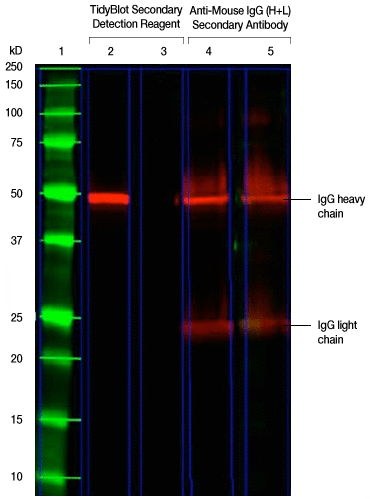
Fig. 2. Detection of the 50 kD SSB protein. TidyBlot Western Blot Detection Reagent:HRP binds exclusively to the native anti-SSB antibody, whereas the HRP-conjugated anti-mouse IgG heavy and light chain secondary antibody recognizes both the native anti-SSB and denatured antibodies (as shown by the detection of heavy and light chains) in this mock IP experiment. HEK293 cell lysate (20 µg) spiked with 3.5 µg of mouse monoclonal antibody of the IgG2b isotype was run in lanes 2, 3, 4, and 5 under reduced conditions for SDS-PAGE and the proteins were transferred onto a PVDF membrane. Precision Plus Protein All Blue Protein Standard was run in lane 1. Mouse anti-SSB PrecisionAb Antibody was used as the primary antibody for the detection of the SSB (also known as Lupus La) protein (~50 kD) in lanes 2 and 4. Lanes 3 and 5 were secondary-antibody-only controls (no primary antibody was added) to determine background staining from the secondary detection reagents. TidyBlot Western Blot Detection Reagent:HRP was used as the secondary detection reagent in lanes 2 and 3. An HRP-conjugated anti-mouse IgG (heavy and light chain) secondary antibody was used for lanes 4 and 5. The blot was visualized with the ChemiDoc MP Imaging System. Please note that TidyBlot Reagent is HRP conjugated and that the western blot bands (red) and protein standards (green) have been pseudocolored.
How do you tackle the problem of visualizing endogenous IgGs when using tissue lysate samples?
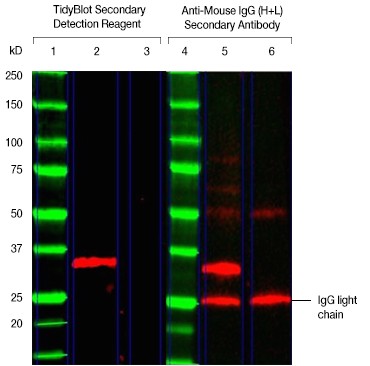
Fig. 3. TidyBlot Reagent helps avoid visualization of additional bands from endogenous IgG binding. TidyBlot Western Blot Detection Reagent:HRP binds exclusively to the native anti-PCNA antibody, whereas the HRP-conjugated anti-mouse IgG heavy and light chain secondary antibody recognizes endogenous IgGs present in the lysate (as shown by the detection of the light chain) in this western blot of mouse thymus lysate. Mouse thymus lysate (25 µg) was loaded onto a 4–15% Criterion™ TGX Stain-Free™ Protein Gel (lanes 2, 3, 5, and 6) and the proteins were transferred onto a nitrocellulose membrane. Precision Plus Protein All Blue Protein Standard was run in lanes 1 and 4. Mouse anti-PCNA PrecisionAb Antibody was used as the primary antibody. Lanes 3 and 6 were secondary-antibody-only controls (no primary antibody was added) to determine background staining from the secondary detection reagents. TidyBlot Western Blot Detection Reagent:HRP was used as the secondary detection reagent in lanes 2 and 3. An HRP-conjugated anti-mouse IgG (heavy and light chain) secondary antibody was used for detection of proteins in lanes 5 and 6. The blot was visualized with the ChemiDoc MP Imaging System. Please note that TidyBlot Reagent is HRP conjugated and that the western blot bands (red) and protein standards (green) have been pseudocolored.[/column]
The TidyBlot Reagent works across a broad range of species and detects both monoclonal and polyclonal antibodies and makes background-free western blotting a reality.
For more information and to view the FAQs, visit https://www.abdserotec.com/tidyblot.
The primary antibody used in western blotting can cause considerable detection of background signals, requiring several optimization steps to reduce the background. For extensively validated western blotting antibodies that eliminate the need for time-consuming optimization to reduce background, explore the PrecisionAb Antibodies at https://www.abdserotec.com/validated-western-blotting-antibodies-precisionab.html.

