Ligand binding to the epidermal growth factor receptor (EGF R) induces receptor homo- and/or heterodimerization and the subsequent phosphorylation of specific tyrosine residues in the cytoplasmic domain of the EGF R. The phosphorylated receptor then recruits adaptor proteins and enzymes to transmit signals from the receptor through signaling pathways to the nucleus, regulating diverse biological functions such as cell proliferation, differentiation, migration, and apoptosis (Morandell et al. 2008; Yarden and Sliwkowski 2001).
More than one hundred proteins have been reported in the literature to interact with EGF R (Morandell et al. 2008). The following summary describes major EGF R tyrosine phosphorylation sites, as well as selected adaptors and signaling proteins.
The majority of tyrosine sites of the EGF R are autophosphorylated. Tyr920 phosphorylation of EGF R acts as a docking site for the phosphatidylinositol 3-kinase regulatory subunit alpha (p85) (Stover et al. 1995). Tyr992 phosphorylated EGF R binds 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1 (PLCγ) (Rotin et al. 1992), resulting in activation of PLCγ-mediated downstream signaling (Emlet et al. 1997). This site is dephosphorylated by the tyrosine-protein phosphatase non-receptor type 11 (SHP2) (Agazie and Hayman 2003). In addition, the guanine nucleotide exchange factor (VAV2) SRC homology 2 domain preferentially binds to the Tyr992 site (Tamas et al. 2003). The E3 ubiquitin ligase, CBL, binds to Tyr1045 upon its phosphorylation (Levkowitz et al. 1999) and mediates its monoubiquitination, which results in receptor endocytosis followed by endosomal/lysosomal sorting (Grøvdal et al. 2004; Haglund and Dikic 2012). Phosphorylation of Tyr1068 and Tyr1086 is required for the binding of growth factor receptor-bound protein 2 (GRB2) adaptor protein (Yamauchi et al. 1997; Rojas et al. 1996; Okutani et al. 1994), which is the key player in EGF-dependent Ras activation (Sebastian et al. 2006). Tyr1068 of EGF R also provides a docking site for the SH2 domain of signal transducer and activator of transcription 3 (STAT3) (Shao et al. 2003). Docking protein (Dok-R) binds EGF R phosphorylated at Tyr1086 and Tyr1148 (Jones and Dumont 1999). The phosphorylated EGF R Tyr1148 and Tyr1173 residues can recruit SHC (Okabayashi et al. 1994). Tyr1148 also binds PLCγ (Chattopadhyay et al. 1999) and VAV2 (Tamas et al. 2003) (Figure 1).
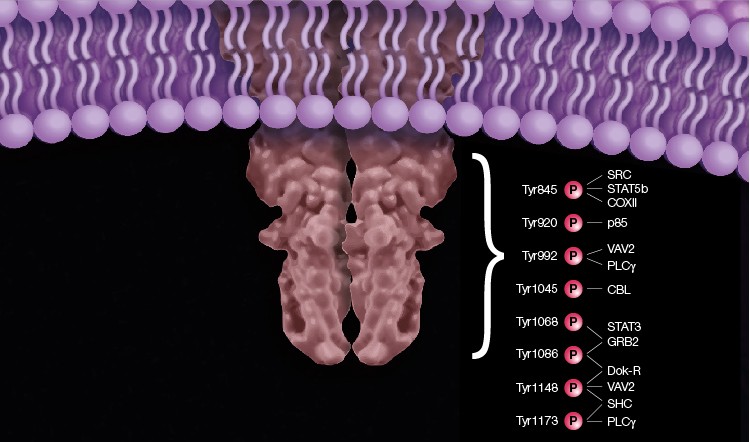
Fig. 1. Schematic representation of the major EGF R tyrosine phosphorylation sites. The phosphorylated receptor recruits adaptor proteins that transmit the activation signal from the receptor to different signaling pathways.
Although the major tyrosine sites of the EGF R are autophosphorylated, some are phosphorylated by intracellular tyrosine kinases (Sebastian et al. 2006). As an example, EGF R Tyr845 is phosphorylated by the proto-oncogene tyrosine-protein kinase (SRC) (Sato et al. 1995). This phosphorylation is required for EGF-induced signal transduction and activation of transcription factor 5B (STAT5b) in breast cancer cell models (Kloth et al. 2003), and mediation of EGF R binding to the mitochondrial protein cytochrome c oxidase subunit II (CoxII) (Boerner et al. 2004).
See our range of phosphospecific antibodies.
References
Agazie YM and Hayman MJ (2003). Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol 23, 7,875–7,886.
Boerner JL et al. (2004). Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol 24, 7,059–7,071.
Chattopadhyay A et al. (1999). The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J Biol Chem 274, 26,091–26,097.
Emlet DR et al. (1997). Subsets of epidermal growth factor receptors during activation and endocytosis. J Biol Chem 272, 4,079–4,086.
Grovdal LM et al. (2004). Direct interaction of CBI with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res 300, 388–395.
Haglund K and Dikic I (2012). The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci 125, 265–275.
Jones N and Dumont D (1999). Recruitment of Dok-R to the EGF receptor through its PTB domain is required for attenuation of Erk MAP kinase activation. Curr Biol 9, 1,057–1,060.
Kloth MT et al. (2003). STAT5b, a Mediator of Synergism between c-SRC and the Epidermal Growth Factor Receptor. J Biol Chem 278, 1,671–1,679.
Levkowtiz G et al. (1999). Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-CBIl/Sli-1. Mol Cell 4, 1,029–1,040.
Morandell S et al. (2008). Quantitative proteomics and phosphoproteomics reveal novel insights into complexity and dynamics of the EGFR signaling network. Proteomics 8, 4,383–4,401.
Okabayashi Y et al. (1994). Tyrosines 1148 and 1173 of activated human epidermal growth factor receptors are binding sites of Shc in intact cells. J Biol Chem 269, 18,674–18,678.
Okutani T et al. (1994). Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells. J Biol Chem 269, 31,310–31,314.
Rojas M et al. (1996). Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem 271, 27,456–27,461.
Rotin D et al. (1992). SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J 11, 559–567.
Sato K et al. (1995). c-SRC phosphorylates epidermal growth factor receptor on tyrosine 845. Biochem Biophys Res Commun 215, 1,078–1,087.
Sebastian S et al. (2006). The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta 1766, 120–139.
Shao H et al. (2003). Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res 63, 3,923–3,930.
Stover DR et al. (1995). SRC phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with SRC and P85 alpha. J Biol Chem 270, 15,591–15,597.
Tamas P et al. (2003). Mechanism of epidermal growth factor regulation of VAV2, a guanine nucleotide exchange factor for Rac. J Biol Chem 278, 5,163–5,171.
Yamauchi T et al. (1997). Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature 390, 91–96.
Yarden Y and Sliwkowski MX (2001). Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2, 127–137.

