Mixed-mode resins are often used when unimodal resins fall short of providing the required process productivity and/or process economics. But not all mixed-mode resins are created equal. For example, the existing selection of available hydrophobic anion exchange resins provides either the needed yield or the needed purity, reducing process productivity either way.
To overcome this deficiency, Bio-Rad developed the Nuvia aPrime 4A Resin with an optimal balance of ion exchange and hydrophobic interactions to deliver simultaneous purity and yield of therapeutic proteins and monoclonal antibodies that are typically difficult to purify. Summarized below are the advantages provided by the Nuvia aPrime 4A Mixed-Mode Hydrophobic Anion Exchange Resin.
Optimal Productivity — Simultaneous Yield and Purity
Nuvia aPrime 4A yields better performance during biomolecule purification in both bind-elute and flow-through (FT) modes.
Bind-Elute Purification
The efficiency of two commercial mixed-mode resins and Nuvia aPrime 4A was tested for the purification of mAb S (approximate pI of 6.9) from a UNOsphere SUPrA eluate containing about 25% aggregated mAb. Only Nuvia aPrime 4A Resin produced a mAb S elution at the mild pH of 6.0 with a monomer recovery of about 90% (Figure 1A). The two other resins showed elution of the antibody at only pH 4.0 and with significant contamination from dimers and aggregates (Figures 1B and 1C).
A. Nuvia aPrime 4A
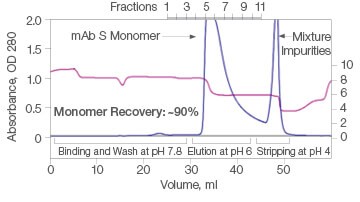
| Sample | Monomer, % | Dimer, % |
Tetramer, % |
| Load | 93.1 | 6.5 | 0.4 |
| Eluate | 100 | ND | ND |
| Strip | 76.8 | 23.2 | ND |
B. Capto adhere
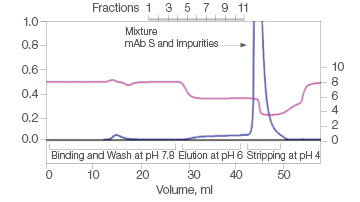
| Sample | Monomer, % | Dimer, % |
Tetramer, % |
| Load | 68.4 | 24.8 | 6.8 |
| Eluate | Target not eluted at pH 6 | ||
| Strip | 66.3 | 26.7 | 7.1 |
C. Capto adhere ImpRes
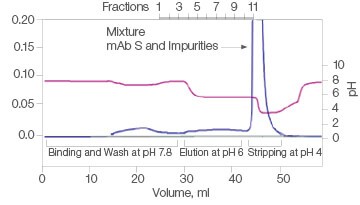
| Sample | Monomer, % | Dimer, % |
Tetramer, % |
| Load | 77.1 | 18.0 | 4.9 |
| Eluate | Target not eluted at pH 6 | ||
| Strip | 77.5 | 18.2 | 4.3 |
Fig. 1. Elutions of mAb S at pH 6.0. A, with Nuvia aPrime 4A, monomer recovery reaches 90%. B and C, with Capto adhere and Capto adhere ImpRes, mAb S is not eluted at pH 6.0. Bound mAb S is stripped from the column at pH 4.0 in a mixture with dimers and aggregates. OD 280 (—); pH(—).
Flow-Through Purification
A DOE study showed that Nuvia aPrime 4A offers better monomer recovery than both the Capto adhere Media for the purification of a basic protein (approx. pI of 8.4) at the optimal FT operation pH of 6.5. Tailing of the FT peak was observed from both Capto adhere Columns but not the Nuvia aPrime 4A Column. A spike at 280 nm was also seen when the Capto adhere Columns were washed with additional equilibration buffer for recovering target protein monomer. Nuvia aPrime 4A Resin’s superior performance is the result of its optimized pore structures and surface properties, which offer efficient mass transfer and minimal nonspecific interaction between biomolecules and this chromatography media (Figure 2).
Fig. 2. Chromatograms from the respective 1 ml columns with monomer recovery and remaining HCP content indicated. FT conditions: 99% monomer content in sample load, 150–200 ng/mg starting HCP content, 20 mM NaPi, 100 mM NaCl, pH 6.5. OD 280 (—); pH (—).
Straightforward Method Development
The mixed-mode nature of the Nuvia aPrime 4A ligand facilitates straightforward method development and process optimization. A simple design of experiment protocol can also be used by method developers to optimize their process conditions. We tested the ease of method development for purification of a basic protein with a pI of ~8.45. Our goal was to find the optimal conditions for the highest monomer recovery and purity. A fractional factorial screening design with two center points was performed. Binding capacity was low under all tested conditions (Figure 3A), suggesting that flow-through purification was more appropriate for this protein. Figures 3B and 3C show the high molecular weight impurity clearance and monomer recovery in response to purification buffer composition.
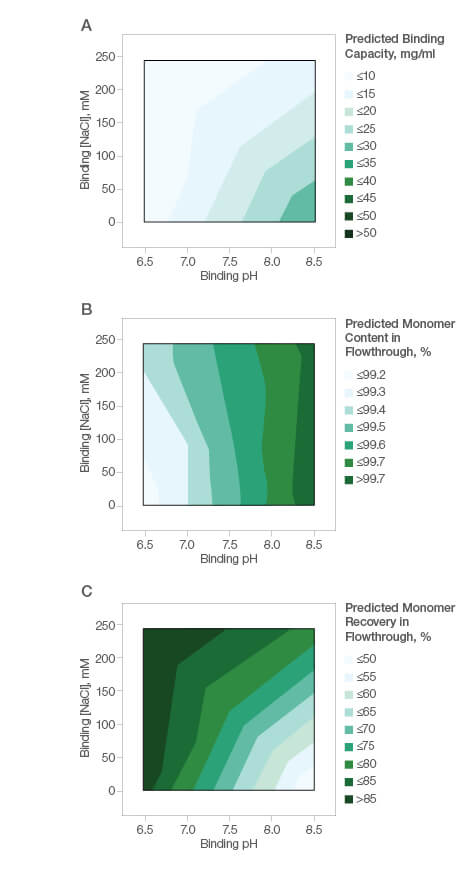
Fig. 3. Basic protein (pI ~8.45) purification using Nuvia aPrime 4A Resin. A, effect of binding buffer pH and NaCl on the binding of the basic protein by Nuvia aPrime 4A; B, effect of binding buffer pH and NaCl on monomer content in the flowthrough fraction; C, effect of binding buffer pH and NaCl on monomer recovery in the flow-through fraction.
Excellent Pressure Flow Properties
Nuvia aPrime 4A is designed with a bead size optimized to achieve process-scale purification of biomolecules at high flow rates without being limited by column pressure. This also provides an increase in process productivity. The column pressure remains below 3 bar up to a linear velocity of 350 cm/hr (Figure 4). Nuvia aPrime 4A Resin’s fast mass transfer dynamics ensure efficient chromatography at these high flow rates, making it an ideal choice for manufacturing purifications.
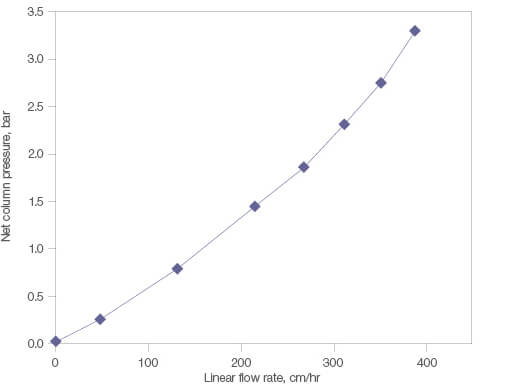
Fig. 4. Pressure-flow performance of Nuvia aPrime 4A Resin. Nuvia aPrime 4A Resin slurry prepared in 1x PBS, pH 7.5 was packed into a 20 x 20 cm column by axial compression at 300 cm/hr with a compression factor of 1.25.
Optimal Process Economics — Robust Performance
Apart from the simultaneous high yield and purity during purification, Nuvia aPrime 4A also has high tolerance to a broad range of pH and salt conditions, making it compatible with most feed streams. This enables capture and polish purification without extensive feedstream conditioning, saving process time and cost.
In addition, Nuvia aPrime 4A is built on a mechanically and chemically stable base bead. This allows the resin to perform consistently with minimal changes to dynamic binding capacity or recovery even after multiple exposures to NaOH (Figure 5).
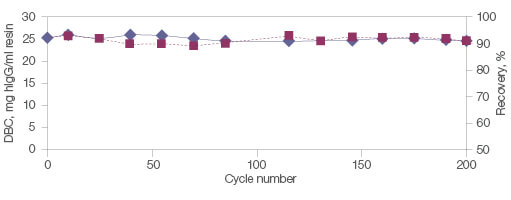
Fig. 5. Stability, reusability, and recovery with Nuvia aPrime 4A Resin. Polyclonal hIgG (1.0 mg/ml) in equilibration buffer (50 mM Na2PO4, pH 8.0) was loaded onto a 1 ml Bio-Scale Mini Column (0.56 x 4 cm) packed with Nuvia aPrime 4A. The column was operated at 150 cm/hr. hIgG was eluted with 100 mM sodium citrate, pH 3.0. The column was cleaned in place for 30 min with 1 M NaOH. The 10% DBC and recovery of hIgG at linear velocity of 150 cm/hr was determined after every ten cycles. DBC (—♦—); recovery (—■—). DBC, dynamic binding capacity; hIgG, human IgG.
Different Formats for Easy Scalability
Nuvia aPrime 4A is available in multiple user-friendly formats, including prepacked Foresight Columns and Plates for purification condition screening and bulk bottles for pilot- to manufacturing-scale purifications. In addition, it is backed by our regulatory support documentation and security of supply commitment.
In summary, Nuvia aPrime 4A offers the following applications and advantages during downstream process purification:
- Provides simultaneous purity and yield for efficient bioprocessing
- Removes multiple impurities, such as viruses, host cell proteins (HCPs), and aggregates and nucleic acids in a single chromatography step
- Enables capture and polish purification without extensive feedstream conditioning
- Can be used to purify both established therapeutic proteins and diverse new constructs in development, including those which lack affinity handles
- Effective in the purification of salt- and pH-sensitive proteins with a high propensity for aggregation and/or degradation
- Offers a wise design space (pH and salt stability) to accommodate the conditions required for difficult-to-purify proteins
- Allows straightforward method development
View detailed technical features of the Nuvia aPrime 4A Resin.
Screen this resin for your application. Request a sample.