Abstract
Surface plasmon resonance (SPR) biosensors analyze biomolecular interactions in a label-free and real-time manner. The ProteOn XPR36 protein interaction array system is an SPR platform for analyzing label-free biomolecular interactions (described in detail in the Applications & Technologies portion of the Bio-Rad site). The ProteOn liposome capturing kit used with the system provides a novel hydrophilic surface chemistry that allows for advanced applications such as drug-lipid interaction analysis. This kit can be used to evaluate small molecule drug delivery by measuring properties of drugs and assessing drug carrier systems. This technote presents the ability of the ProteOn liposome capturing kit in analyzing liposome-small molecule interactions and shows a comparison of lipophilic and hydrophilic lipsome surface chemistries.
Introduction Results Conclusion References
Figures
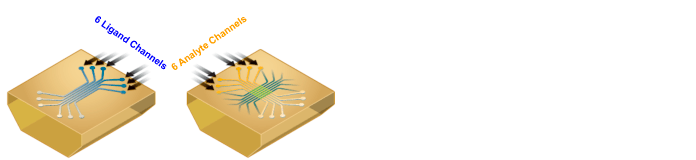
The 6 x 6 biomolecular interaction array of the ProteOn system

Fig. 1. The configuration of the 6 x 6 biomolecular interaction array.Screening of the Interactions Between Small Molecule Drugs and Liposomes
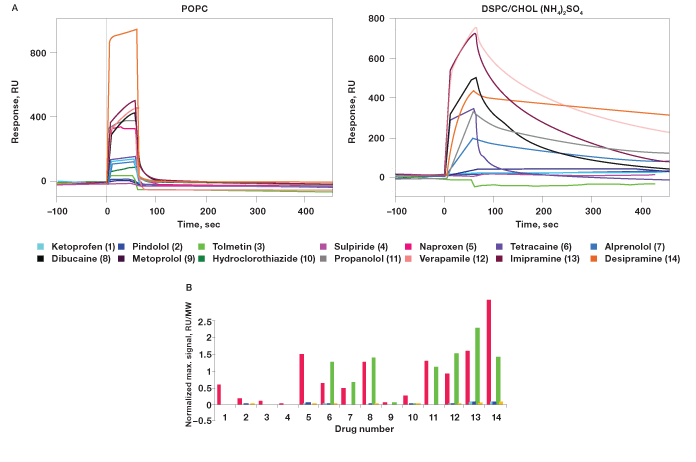
Fig. 5. Sensorgrams and normalized maximum signals of interactions of the drugs and liposomes.
Liposome Capture Using the ProteOn GLC Lipid Kit

Fig. 2. Workflow for liposome capture using the GLC lipid kit, based on conventional lipophilic surface chemistry.Dose Response Analysis of Small Molecules and Drugs
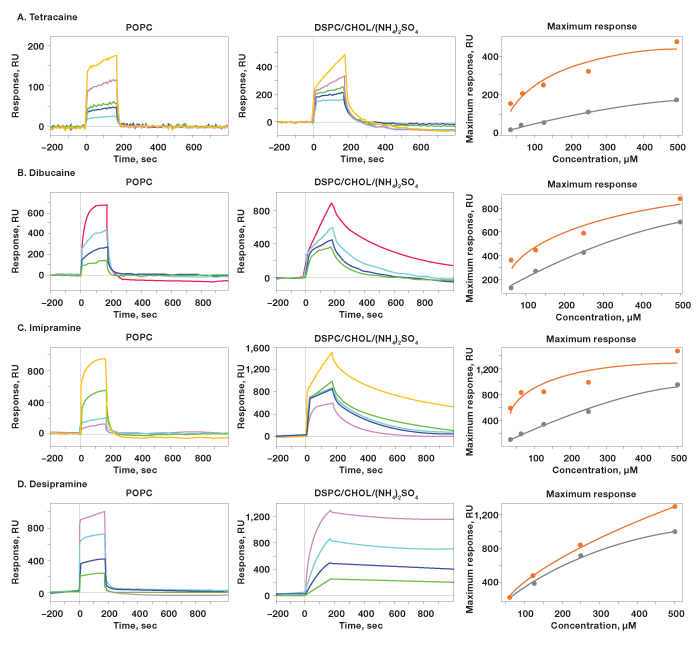
Fig. 6. Dose-response analysis of four small molecule drugs: A, tetracaine; B, dibucaine; C, imipramine; D, desipramine.
Liposome Capture Using the ProteOn Liposome Capture Kit
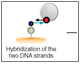
Fig. 3. Workflow for liposome capture using the liposome capturing kit, based on the novel hydrophilic surface chemistry.Comparison of Lipophilic Surface Chemistry vs. Hydrophilic Surface Chemistry
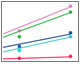
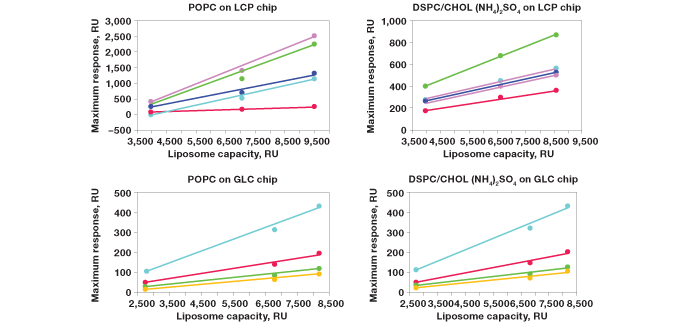
Fig. 7. Correlation of responses with the surface density of liposomes.
Experimental Design for Screening of the Interactions Between Small Molecule Drugs and Liposomes
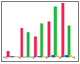
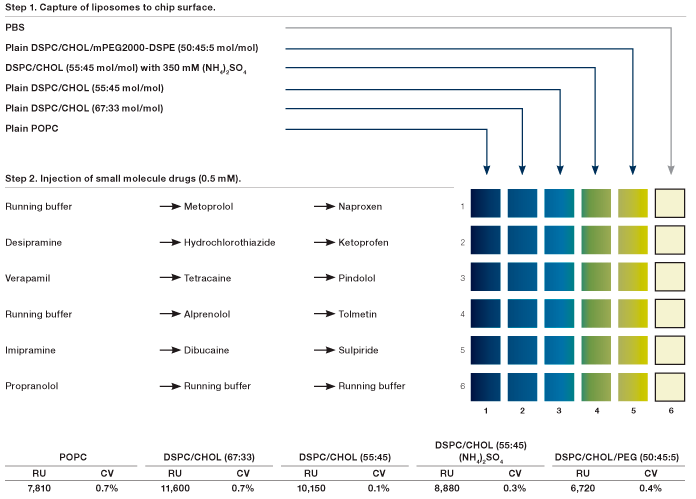
Fig. 4. The experimental design of the screening of fourteen small molecule drugs against five liposomes using the 6 x 6 configuration in the ProteOn XPR36 system.
Introduction
The ProteOn XPR36 system is an SPR platform that allows for highly efficient optimization of experimental conditions by featuring an array of 6 x 6 biomolecular interactions. The array allows simultaneous probing of six target immobilization conditions in six ligand channels together with six interaction conditions in six analyte channels, shown in Figure 1. The 6 x 6 configuration also enables novel referencing options for high-quality data and high-throughput sample analysis. These advantages position the ProteOn XPR36 system as an optimal SPR platform in label-free drug discovery applications, providing high-quality data, ideal experimental versatility, and excellent cost-effectiveness. Recently, in addition to the traditional protein-protein interaction applications, the ProteOn XPR36 system has contributed to the rapid progression made in exploring novel applications, such as drug-lipid interaction analysis.
Fig. 1. The configuration of the 6 x 6 biomolecular interaction array. The interactions are realized through the formation of six ligand channels followed by six perpendicular analyte channels on the surface of a sensor chip. The 6 x 6 configuration enables highly efficient optimization of experimental conditions, novel referencing options for high data quality, and high-throughput sample analysis.
Results
ProteOn Lipid Application Kits
Lipid vesicles such as liposomes have been applied in SPR biosensors for analyzing cell membraneinvolved biomolecular interactions. Liposomes can be used to construct a lipid bilayer environment, thus this approach facilitates the characterization of cell membranerelated biological factors, such as the partition of small molecule drugs across cell membranes.
In order to apply liposomes in SPR biosensors, a method must be used to capture liposomes on the surface of sensor chips. The conventional method is based on a lipophilic surface chemistry, such as covalently linking alkyl groups to the chip surface to anchor liposomes through lipophilic interactions. Sensor chips or kits now on the market that use this method include the ProteOn GLC lipid kit (Bio-Rad Laboratories, Inc.) and the Biacore L1 sensor chip (GE Healthcare). The workflow for application of the GLC lipid kit is shown in Figure 2. This method is effective in many liposome-based interaction analysis cases. However, its applications are limited by the drawbacks, which include the risk of liposome structure rapture, incomplete surface regeneration, and nonspecific binding of analytes to the lipophilic surface in the following interaction analysis step.
Fig. 2. Workflow for liposome capture using the GLC lipid kit, based on conventional lipophilic surface chemistry.
The ProteOn liposome capturing kit offers a novel method for liposome capture. This kit includes the LCP sensor chip and the LCP capturing reagent kit. The reagent kit offers a uniquely designed cholesterol-tagged, uneven, double-stranded DNA that binds to liposomes through lipophilic interaction. The attachment to the liposomes is very stable, which is attributed to the bivalent binding of the two cholesterol moieties. The uneven double-stranded DNA then binds to a single-stranded DNA on the chip surface to anchor the liposomes. Thus, this kit provides a hydrophilic surface chemistry that allows for high-performance liposome capture. The benefits of this method include (1) reduction of nonspecific binding of analytes to the lipophilic chip surface, (2) easy and effective chip surface regeneration by triggering DNA dehybridization, and (3) ability to maintain captured liposomes intact using the double-stranded DNA linker on the chip surface. The workflow for applying the liposome capturing kit is illustrated in Figure 3. Using this kit, it is also possible to form multiple layers of liposomes if high-level liposome capture is desired.
Fig. 3. Workflow for liposome capture using the liposome capturing kit, based on the novel hydrophilic surface chemistry. The LCP chip surface is saturated with single-stranded biotinylated DNA molecules, and liposomes tagged with cholesterol-labeled double-stranded DNA molecules are captured to the surface through DNA hybridization.
LiposomeSmall Molecule Drug Interaction Analysis Used as a Drug Delivery Model
Small molecule drugs with ideal efficacy should be both soluble in aqueous solution and able to pass through lipophilic matter such as cell membranes. The balance of hydrophilicity and lipophilicity is an essential aspect in drug delivery because it indicates the drugs intestinal permeability and efficacy to intracellular targets of small molecule drugs.
Thus, in order to optimize that balance during the drug discovery process, many in vitro methods to analyze the drug partitioning across cell membranes have been proposed, such as partition coefficient analysis using an equilibrated system of aqueous solution and n-octanol (Buchwald and Bodor 1998). However, these methods do not establish an interface of aqueous solution and lipid bilayer structure, thus they may not be able to accurately predict drug transport across biological membranes. Researchers have been analyzing drug partitioning using liposomes, which accurately mimic the lipid bilayer geometry and ionic characteristics of cell membranes, allowing a good correlation to in vivo analysis of intestinal permeability of small molecule drugs (Balon et al. 1999). In this report, a variety of liposomes were captured on a sensor chip surface using the ProteOn liposome capturing kit, and small molecule drugs were screened against these liposomes to assess their partitioning characteristics across cell membranes of different types.
Another common application of liposomes is as a drug carrier system to increase the therapeutic index of the delivered drugs. Liposomes filled with ammonium sulfate are an efficient system for active loading of weakly basic small molecule drugs. The formed transmembrane ammonium sulfate gradient creates an acidic pH environment inside the liposomes while the outside bulk solution is at a more neutral pH. When the neutral form (not protonated) of a small molecule drug penetrates into the liposomes, it will become protonated and lose the ability to penetrate the lipid bilayer membranes. Therefore, if the pH inside the liposomes is low enough to protonate basic drugs, and the pH outside the liposomes is likewise higher, the drug will accumulate inside the liposomes (Barenholz 2006).
As a label-free SPR biosensor, the ProteOn XPR36 system is able to directly analyze the interactions between small molecule drugs and liposomes, serving as an ideal platform for the liposome-based in vitro characterization of drug partitioning and drug carrier systems (Danelian et al. 2000, Baird et al. 2002).
Screening of the Interactions Between Small Molecule Drugs and Liposomes
Using the 6 x 6 configuration in the ProteOn XPR36 system, five different types of liposomes were captured on the surfaces of the vertical channels (ligand channels) by applying the ProteOn liposome capturing kit as instructed in the product manual (www.bio-rad.com/lcpkit). Fourteen small molecule drugs were injected in the horizontal channels (analyte channels) in three consecutive steps to screen the interactions between the drugs and the liposomes. The experimental design is shown in Figure 4. The sensorgrams of the interaction analysis are referenced to a blank surface (either a blank ligand channel or interspots) and a blank injection (simultaneous buffer injection in an analyte channel) (Bio-Rad Bulletin 5390). The screening results are shown in Figure 5.
Fig. 4. The experimental design of the screening of fourteen small molecule drugs against five liposomes using the 6 x 6 configuration in the ProteOn XPR36 system. Five liposomes: plain POPC, plain DSPC/CHOL 67:33 mol/mol, plain DSPC/CHOL 55:45 mol/mol, DSPC/CHOL 55:45 mol/mol with 350 mM (NH4)2SO4 (DSPC/CHOL (NH4)2SO4 in abbreviation), and plain DSPC/CHOL/mPEG2000-DSPE 50:45:5 mol/mol. Running buffer: 50 mM NaH2PO4 at pH 5.5, 150 mM NaCl, 0.1% DMSO. Abbreviations: PBS phosphate-buffered saline; POPC 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; DSPC 1,2-distearoyl-sn-glycero-3-phosphocholine; CHOL cholesterol; mPEG2000-DSPE 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]. Flow rate was 30 l/min for liposome capture and 100 l/min for analyte injection. The capture levels of the five liposomes and the colume volume (CV) among six interaction spots in each ligand channel are shown in the table. RU, response units.
Fig. 5. Sensorgrams and normalized maximum signals of interactions of the drugs and liposomes. A, Sensorgrams obtained from the ligand channels of POPC and DSPC/CHOL (NH4)2SO4. Weak or no responses were observed in the other ligand channels, indicating no interactions between the small molecule drugs and the other three liposomes. B, Normalized maximum signals of the interactions of the drugs and the liposomes. Responses were normalized by dividing by the compounds MW. Strong responses were observed when using POPC liposomes and ammonium sulfate gradient DSPC/CHOL (NH4)2SO4 liposomes. POPC (); DSPC/CHOL (67/33) (); DSPC/CHOL (55/45) (); DSPC/CHOL (NH4)2SO4 (); DSPC/CHOL/PEG (50/45/5) (). RU, response units; MW, molecular weight.
The seven compounds that bound to DSPC/CHOL (NH4)2SO4 are weak bases (Drug numbers 6, 7, 8, 11, 12, 13, and 14). The three weak acids did not show binding to this ammonium liposome (Drug numbers 1, 3, and 5). This result is consistent with the theory that weakly basic small molecule drugs are retained in the acidic environment inside the ammonium sulfate gradient liposomes. Four drugs that showed strong interactions with POPC and DSPC/CHOL (NH4)2SO4 were chosen for further dose-response analysis: tetracaine, imipramine, dibucaine, and desipramine.
The four small molecule drugs were prepared in a twofold dilution series ranging from 500 to 31 M and injected in the analyte channels. The interactions on the 6 x 6 array were measured and the sensorgrams obtained from the ligand channels of POPC and DSPC/CHOL (NH4)2SO4 are shown in Figure 6. The maximum responses in the sensorgrams are plotted against the analyte concentrations and the following facts are observed: In most cases, biphasic binding was observed, indicating complex binding mechanisms that may have occurred close to the penetration of drugs into the liposomes. The term biphasic binding means that the observed interaction is composed of fast and slow binding mechanisms. Different interaction patterns were observed between the two liposomes: POPC showed fast association and fast dissociation, while DSPC/CHOL (NH4)2SO4 showed biphasic binding and much slower dissociation. In some cases, only the slow phase is observed. This is in agreement with the active loading mechanism accumulation of drug molecules inside the liposomes when the ammonium gradient is formed.
The results demonstrate that this drug delivery model can be used for the evaluation of drug partitioning across cell membranes.
Fig. 6. Dose-response analysis of four small molecule drugs: A, tetracaine; B, dibucaine; C, imipramine; D, desipramine. The sensorgrams were obtained from the ligand channels of POPC and DSPC/CHOL (NH4)2SO4. The two liposomes were captured to the level of 7,800 RU and 8,900 RU, respectively. The small molecule drugs were injected in a twofold dilution series ranging from 500 M to 31 M. The maximum responses in the sensorgrams are plotted against the analyte concentrations for each liposome-drug interaction. DSPC/CHOL (NH4)2SO4 (); POPC (). RU, response units.
In order to further characterize the liposomesmall molecule interactions in this drug delivery model, the correlation between maximum responses in analyte injections and liposome surface densities was investigated. Surfaces with two types of liposomes, POPC and DSPC/CHOL (NH4)2SO4, were employed. Multiple small molecule drugs were used as analytes. In addition, the two surface chemistries for capturing liposomes the conventional lipophilic surface chemistry (by ProteOn GLC lipid kit) and the novel hydrophilic surface chemistry (by ProteOn liposome capturing kit) were compared. The results are shown in Figure 7. When the lipophilic surface chemistry was applied, a GLC sensor chip was used and the variation of liposome surface densities was realized by changing the surface lipophilicity. When the hydrophilic surface chemistry was applied, an LCP sensor chip was used and the variation of liposome surface densities was realized by using one, two, or three layers of liposomes. The linearity between maximum responses in analyte injections and liposome surface densities is observed in all cases.
Fig. 7. Correlation of responses with the surface density of liposomes. Different liposome surface densities were prepared on the two surface chemistries, using the ProteOn liposome capturing kit and the ProteOn GLC lipid kit. Two liposomes, POPC and DSPC/CHOL (NH4)2SO4, were captured on chip surfaces and multiple small molecule drugs were injected at the concentration of 0.5 mM. The injection time was set at 180 sec, and the flow rate for liposome capture was 25 l/min. The linearity between maximum responses and liposome surface densities is shown. alprenolol 0.5 mM (); propanolol 0.5 mM (); dibucaine 0.5 mM (); imipramine 0.5 mM (); despiramine 0.5 mM (); verapamil 0.5 mM (). RU, response units.
While both the lipophilic and hydrophilic surface chemistries showed very good performance in the liposomesmall molecule interaction analysis, the absolute values of responses in analyte injections were significantly different between the GLC and LCP sensor chips. At similar liposome surface densities, most absolute values of responses are several-fold higher on the LCP sensor chip using the ProteOn liposome capturing kit. An explanation is that the DNA linker between liposomes and the chip surface reduces the steric hindrance to access the entire surface of a liposome particle. Also, the hydrophilic surface chemistry is beneficial for maintaining intact liposomes, which further guarantees the data quality in applying this drug delivery model. This advantage results in higher performance of the novel hydrophilic surface chemistry compared with the conventional liposome capture method.
Conclusion
The application results demonstrate excellent performance of the ProteOn liposome capturing kit in the label-free analysis of liposomesmall molecule interactions. The novel hydrophilic surface chemistry for liposome capture shows excellent performance in controlling liposome surface densities and maintaining accurate interaction analysis. Utilizing the One-shot Kinetics approach, dose-response sensorgrams were successfully and efficiently obtained for further characterization. The comparison with the conventional liposome capture method positions this novel method as an advanced solution for liposome applications in SPR biosensors. Thus, the combination of the novel surface chemistry and the ProteOn XPR36 system is able to extend SPR technology to the liposome-based small molecule drug delivery analysis.
References
Baird CL et al. (2002). Surface plasmon resonance characterization of drug/liposome interactions, Anal Biochem 310, 9399.
Balon K et al. (1999). Drug liposome partitioning as a tool for the prediction of human passive intestinal absorption,
Pharm Res 16, 882888.
Barenholz Y (2006). Amphipathic weak base loading into preformed liposomes having a transmembrane ammonium ion gradient: from the bench to approved Doxil. Liposome Technology, Vol II (3rd Edition) 2, 125.
Buchwald P and Bodor N (1998). Octanol-water partition: searching for predictive models, Curr Med Chem 5, 353380.
Danelian E et al. (2000). SPR biosensor studies of the interaction between 27 drugs and a liposome surface: correlations with fraction absorbed in humans, J Med Chem 43, 20832086.
Legal Notices
Biacore is a trademark of GE Healthcare.
The ProteOn XPR36 protein interaction array system is covered by Bio-Rad patents, including United States patent numbers 8,111,400, 8,105,845, 7,999,942, and 7,443,507.
This product or portions thereof is manufactured and sold under license from GE Healthcare under United States patent numbers 5,492,840, 5,554,541, 5,965,456, 7,736,587, and 8,021,626, and any international patents and patent applications claiming priority.