The rapid development of vaccines against SARS-CoV-2 has been an incredible achievement with undeniable impact. But the question remains: How long does vaccine-induced immunity last? Measuring isotype-specific antibodies against SARS-CoV-2 and the effectiveness of neutralizing antibodies against variants can help us better understand the duration and breadth of the humoral response. These data are critical to aid public health researchers and inform development decisions for vaccines and therapeutics.
The ongoing COVID-19 pandemic has resulted in a rise of SARS-CoV-2 variants of interest and concern and has enabled these (often more transmissible or infectious) versions of the virus to spread around the globe. The availability of vaccines and the increase in vaccination rates have greatly reduced the risk of becoming severely ill or dying from COVID-19, but questions remain about the duration and effectiveness of the immune response from natural infection or vaccination against these variants.
Measuring antibodies against SARS-CoV-2 and the ability of neutralizing antibodies to inhibit infection with these variants is one way to assess immunity. Studies are being conducted by researchers involved in public health, vaccine development, and COVID-19 therapeutic development. These studies often use traditional, cell-based assays to determine virus neutralization capabilities of antibodies in samples.
Traditional Neutralization Assays
One such cell-based bioassay is the plaque reduction neutralization test (PRNT). However, this assay requires a biosafety level (BSL) 3 lab and skilled technicians. The pseudotyped virus neutralization test (pVNT), on the other hand, can be run in a BSL 2 lab, but still requires the construction of a plasmid and transfection of a stable cell line before the neutralization assay can be run.
In addition to these practical constraints, PRNT and pVNT bioassays take several days to complete and are only able to measure neutralization antibodies against one wild-type or variant protein subunit at a time. Enzyme-linked immunosorbent assays (ELISAs) have higher throughput, thanks to the 96-well plate format, but can still only assay for neutralizing antibodies against one viral antigen per well.
Increasing Throughput with Bio-Plex Assays
To increase throughput, Bio-Rad has developed the quantitative, research use only (RUO) Bio-Plex Pro Human SARS-CoV-2 Variant Neutralization Antibody 11-Plex Panel. The kit can be used to measure concentrations and percent inhibition of neutralizing antibodies against two wild-type and nine variant SARS-CoV-2 antigens in a single well.
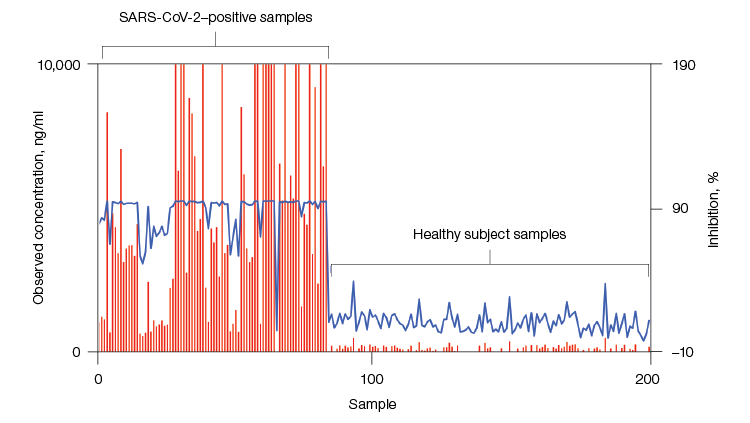
Plot comparing the degree of receptor binding domain (RBD) antibody neutralization (percentage inhibition) and observed concentration in samples. Left, samples positive (n = 84) for SARS-CoV-2 (confirmed by PCR testing); right, control samples from healthy subjects (n = 118). Observed concentration is shown in red; percentage inhibition is shown in blue.
These highly precise multiplex immunoassays can efficiently answer the question of whether the neutralizing antibodies in samples from different subjects are able to effectively inhibit the interaction of the viral antigens (coated on xMAP beads) with the detection ACE2 receptor. The competitive assay format yields lower median fluorescent intensity (MFI) for samples that have more effective neutralizing antibodies, whereas the inverse is true for sandwich immunoassays.
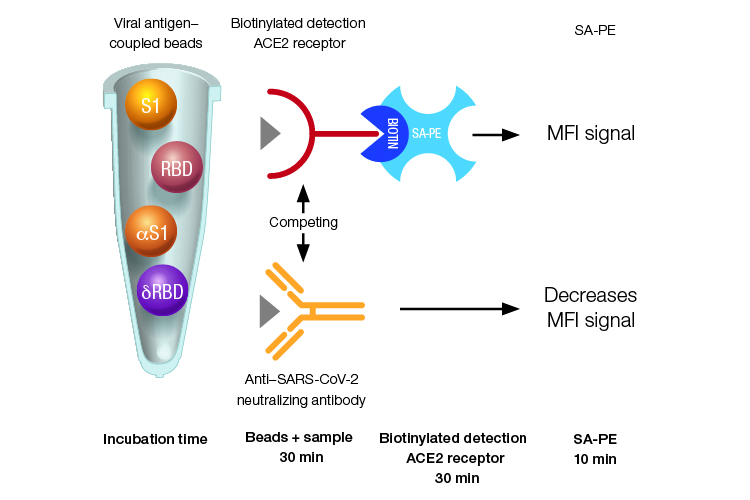
Quantitative* Bio-Plex Pro SARS-CoV-2 competitive assay format and incubation times. Samples are incubated with SARS-CoV-2 antigen–coupled beads for 30 minutes, allowing anti-SARS-CoV-2 neutralizing antibodies in the sample to bind to antigens on the beads. Next, the mixture is incubated for 30 minutes with biotinylated detection ACE2 receptor, which competes with the neutralizing antibodies in the sample for binding of the antigen–coupled beads. All unbound neutralizing antibodies and detection ACE2 receptors are washed away. Finally, a 10-minute incubation with streptavidin-phycoerythrin (SA-PE) enables fluorescent detection. MFI, median fluorescence intensity; δRBD, Delta receptor binding domain; αS1, Alpha spike 1; SA-PE, streptavidin-phycoerythrin.
*The Bio-Plex SARS-CoV-2 Delta RBD and Delta Spike Trimer Assays are qualitative.
The Bio-Plex Pro Human SARS-CoV-2 Variant Neutralization Antibody 11-Plex Panel can assay for neutralizing antibodies against SARS-CoV-2 wild-type receptor binding domain (RBD) and spike 1 (S1) antigens, along with the following variants: Alpha S1, Beta S1, Gamma RBD, Epsilon RBD, Kappa RBD, D614G S1, E484K RBD, K417N RBD, and N501Y RBD.
Flexibility to Fit Your Needs
For maximum customizability, standalone Delta Spike Trimer and Delta RBD 2-Plex Bead mixtures with a positive control, or any standalone variant coupled bead, can be combined with the Bio-Plex Pro Human SARS-CoV-2 Neutralization Antibody 2-Plex Panel, comprised of wild-type RBD and S1. The standalone variant coupled beads can also be run in any combination with a standard, detection ACE2 receptor, and reagent kit to quantify neutralizing antibodies. The MFI can be used to calculate the percentage inhibition, allowing comparisons of the effectiveness of neutralization antibodies against wild-type RBD and S1 versus the different RBD and S1 variants.
These neutralizing antibody assays, along with the Bio-Plex Pro Human IgA, IgG, and IgM SARS-CoV-2 N/RBD/S1/S2 4-Plex Panels, provide high-throughput tools ideal for characterizing the efficacy of the humoral response against SARS-CoV-2 in post-market surveillance studies and efficacy studies in ongoing vaccine and COVID-19 therapeutic development.
The Bio-Plex Pro Human SARS-CoV-2 Variant Neutralization Antibody 11-Plex Panel comes as a ready-to-use, 96-well kit containing premixed beads, a detection ACE2 receptor, standard, positive control, and buffers that produce results for neutralizing antibodies against SARS-CoV-2 wild-type RBD and S1 plus nine variants. Only 15 microliters of sample are required to run the panel in duplicate. Flexible configurations include singleplex assay reagents, which enable measurement of specific neutralization antibodies to any available combination of SARS-CoV-2 variants. The coupled beads can be used to measure antibodies in any species, making the assay useful for both preclinical and clinical trials. The kit is compatible with RUO Bio-Rad platforms, including the Bio-Plex 200 and Bio-Plex 3D Multiplex Immunoassay Systems, as well as all other RUO xMAP platforms.
Visit our website to learn more about Bio-Plex Multiplex SARS-CoV-2 Serology and Neutralization Assays.

