In the highly regulated world of analytical and quality control departments within biopharma and contract research organizations (CROs), maintaining compliance while leveraging advanced digital imaging technologies can be a daunting task. Luckily, Bio-Rad Image Lab Touch Software takes this challenge head-on by offering a seamless solution for digital imaging. Learn how Image Lab Touch Software integrates with Bio-Rad imaging systems to support researchers in meeting stringent regulatory requirements, ensuring data integrity and security without compromising efficiency or ease of use.
Compliance at Your Fingertips: Using Image Lab Touch Software for Digital Imaging in Regulated Environments
Digital imaging has been widely adopted in academic labs, early discovery biotech/biopharma labs, and contract research organizations (CROs) due to its ease of use, streamlined analysis, and broad dynamic range. However, integration in regulated environments has lagged. This delay is likely due to several factors, including the need to revise standard operating procedures (SOPs) and implement additional software enhancements to meet regulatory guidelines such as U.S. FDA 21 CFR Part 11 or EU GMP Annex 11, which require audit trails, user access control, and data integrity. Adhering to these regulatory guidelines is essential for ensuring the integrity, security, and reliability of electronic records, and controlling user access, especially in regulated environments.
Despite these hurdles, the benefits of digital imaging are undeniable. To support regulatory compliance, Bio-Rad’s latest digital imagers — the ChemiDoc, ChemiDoc MP, ChemiDoc Go, and GelDoc Go Imaging Systems — feature touchscreen integration and onboard computing technologies, reducing the need for external computers and optimizing the space for instrumentation. Additionally, advanced software features are now available to assist regulated labs with maintaining comprehensive electronic recordkeeping, user access control, and secure data sets — all in line with their SOPs.
From Image Acquisition to Analysis: End-to-End Compliance Solutions
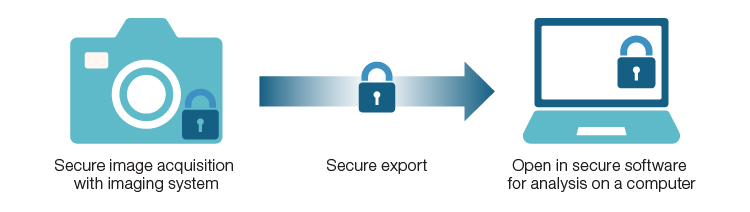
Implementing instrument touchscreens has greatly reduced the need for dedicated computers for instrument operation, as demonstrated by Bio-Rad’s family of digital imagers. These imaging systems feature a touchscreen running Image Lab Touch Software — fully integrated for operation and image acquisition directly on the device. The software’s user-friendly design includes features tailored to assist regulatory environments with audit trails, administrator rights management, and customizable access options.
Key features include:
- Secure password requirements for all users
- Approval process for new users
- Restricted deletion of image acquisitions
- Limited export to USB
- Restricted linking to BR.io cloud platform
- Limited network export locations
- Automatic export to secure network locations
These features support regulatory labs in developing and executing their SOPs, working seamlessly with Bio-Rad’s Image Lab Software, Security Edition. As a PC-based application, Image Lab Software, Security Edition, is tailored for labs working in regulated environments, offering more advanced image analysis and processing options. End-to-end regulatory compliance begins with the secure acquisition of images using Image Lab Touch Software and culminates with enhanced image analysis using Image Lab Software, Security Edition. Together, these tools provide a robust foundation for compliance and advanced imaging workflows, paving the way for integration with Bio-Rad’s cutting-edge imaging systems.
For detailed instructions on incorporating these features in your process, see bulletin 3705.
Paperwork for the Sake of Data Integrity
While maintaining audit trails, instrument logs, and integration into internal data management systems can be difficult to implement initially, these measures are required in regulated settings. To ease this burden on lab administrators, Bio-Rad’s digital imagers have incorporated advanced software features specifically designed to support these critical compliance needs.
These features include:
- Administrator controls: set user permissions and password requirements to manage access and data handling
- Secure file export: ensure data integrity with encrypted file formats, accessible and validated only through Image Lab Software, Security Edition
- Audit trails: log user actions and system operations to maintain traceability and accountability
- Version compatibility and updates: regularly update Image Lab Touch Software and instrument firmware to meet evolving regulatory requirements and software enhancements
- Integration controls: manage data flow to external platforms like the BR.io cloud platform and network locations, preventing unauthorized access and ensuring data security
For a thorough step-by-step process, see bulletin 3727.
Introducing the Bio-Rad Family of Imaging Systems
From DNA and protein gels to chemiluminescence, fluorescence detection, and Stain-Free technology, Bio-Rad’s family of imaging systems are designed to meet the diverse needs of labs, including those in regulatory environments. These systems offer a range of features, from simple image capture to advanced multiplexing (system-dependent), all with high-resolution performance.
ChemiDoc and GelDoc Imaging Systems Overview
- ChemiDoc Go Imaging System
- Modern, compact design with touchscreen operation
- Advanced chemiluminescence and StarBright fluorescence detection (two channels)
- Colorimetric blot detection
- Cloud connectivity
- Energy-efficient
- ChemiDoc MP Imaging System
- Exceptional fluorescence and chemiluminescence detection across multiple channels for enhanced protein quantification
- Multiplexed detection for chemiluminescence, fluorescence, and Stain-Free blot imaging
- ChemiDoc Imaging System
- High sensitivity chemiluminescence
- Upgradable to ChemiDoc MP for multiplex fluorescence detection
- GelDoc Go Imaging System
- Dedicated system for documenting nucleic acid and protein gels
- Stain-Free imaging; colorimetric blot and Coomassie staining detection
- Simple operation with preconfigured protocols for fast imaging
Example Image Acquisition and Analysis

Digital Imaging Is Coming to a Regulated Lab Near You
Bio-Rad’s ChemiDoc, ChemiDoc MP, ChemiDoc Go, and GelDoc Go Imaging Systems, paired with Image Lab Touch and Image Lab Software, Security Edition, offer a comprehensive solution for ensuring data integrity and compliance within SOPs. By integrating advanced imaging systems and regulatory-focused software, regulated labs can confidently generate and manage digital images while ensuring compliance and data integrity. This integration empowers labs to modernize workflows, transitioning from traditional densitometers and colorimetric western blots to cutting-edge, twenty-first-century technology.
Visit our website to learn more about streamlining compliance and improving workflows with Bio-Rad’s imaging solutions.

