Bispecific antibodies (bsAbs) are an important addition to the immuno-oncology toolbox. Designed to recognize two distinct epitopes, bsAbs have enhanced binding, specificity, and efficacy compared to current monovalent antibody therapeutics, making them exciting candidates for more targeted cancer treatments.
bsAbs physically link two different epitopes in a dependency that can be spatial, with binding events occurring simultaneously, or temporal, with binding events occurring sequentially — a novel functionality which broadens the mechanistic range of antibody-mediated therapies, such as linking an effector to a target cell. They come in various formats, ranging from antibody fragments with no Fc region, to large immunoglobulin G (IgG)‒like molecules, and they provide clinicians with an off-the-shelf approach to cancer treatment.
Though their bispecific nature extends efficacy testing workflows, and makes purification and large-scale production more difficult, bsAbs have come a long way since first developed and they are typically cheaper and easier to produce than cell-based therapies.
Broadening the Reach of Immunotherapy
Amgen Inc. is a biotechnology company that has pioneered the use of living cells to produce biologic medicines. Its bispecific T-cell engager (BiTE) platform is an innovative technology designed to engage the immune system against numerous different types and stages of cancers. BiTE molecules offer more versatility in potential cancer treatments than current cell-based therapies, and they are designed to have a short in vitro half-life, meaning that they will be cleared from a patient’s system within several hours. They also eliminate the need to extract T cells for manipulation, expanding the availability and accessibility of immuno-oncology to patients and clinical areas currently unmet by cell-based therapies, such as chimeric antigen receptor T-cell (CAR T-cell) treatment.
Clinical interest in bsAbs gained momentum following the promising data and market success of blinatumomab (BLINCYTO, Amgen), a BiTE, which first received U.S Food and Drug Administration (FDA) approval in 2014 and European Medicines Agency (EMA) approval in 2015. BiTE molecules function by inducing proximity at the cellular level, bringing T cells together with tumor cells so that the former can recognize and destroy the latter. Blinatumomab, for example, is an anti-CD19 x anti-CD3 BiTE molecule, and is currently being investigated for the treatment of acute lymphoblastic leukemia.
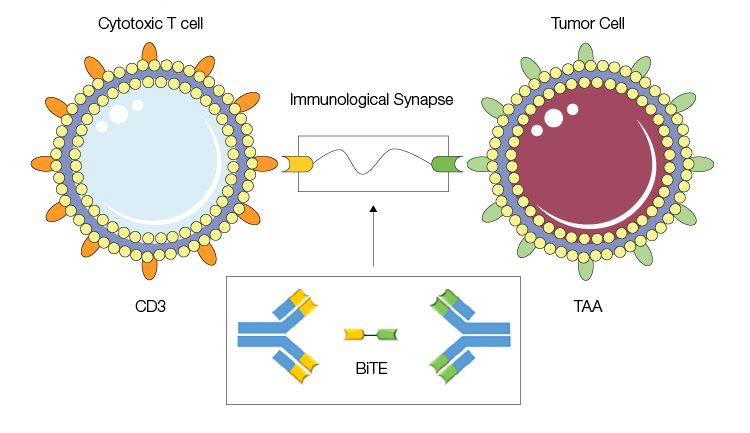
Bispecific T-cell engager (BiTE) molecules bind to CD3 and TAA on T cells and tumor cells, respectively, bringing them into close proximity and triggering destruction of the tumor cells. Image adapted from Kamta et al. 2017 under a CC BY 4.0 license.
Solid tumors have proven difficult to target with current antibody and cell-based therapies, primarily due to immunosuppressive tumor microenvironments and lower antigen expression levels. Designed to target tumor-associated antigens such as EpCAM, HER2, and EGFR, BiTE molecules extend the reach of antibody-mediated therapies against solid tumors by delivering more targeted treatments. In the pipeline, Amgen has BiTE molecules targeting seven types of cancer in clinical trials, and has additional molecules in the design stage (amgenpipeline.com).
Solving Challenges in Bispecific Production and Purification
There are challenges across all stages of drug discovery and development systems, which are further complicated by the bispecific nature of therapeutics bsAbs; workflows for bsAbs are effectively doubled. Rapid, accurate, and highly sensitive screening methods are therefore crucial.
Flow cytometry is a high-throughput screening technique performed directly on antibody-expressing cells, and allows a quick, multiplexed analysis. To improve throughput without comprising data quality, the Bio-Rad ZE5 Cell Analyzer uses robotic automation to enable the analysis of 96-well plates in 15 min and 384-well plates in less than 50 min. This provides both an effective method to rank potential cell lines and an avenue for efficient downstream assays to monitor the effect of bsAbs in vivo. The ZE5 Cell Analyzer is equipped with up to five lasers to support experiments with up to 27 colors for greater panel design flexibility, further strengthening bsAbs screening workflows.
Once a bsAb candidate is selected, a stable cell line must be identified to produce it reliably and at scale for consistent supply. Though advances in genetic engineering have been fundamental in supporting high-producing cell lines, the successful large-scale development of bsAbs hinges on the genetic stability of such lines.
For certainty during characterization and expansions, precise methods for gene copy number assessment are essential. Droplet Digital PCR (ddPCR) technology offers the accuracy, sensitivity, and absolute quantification needed for gene edit confirmation in cell line development. With Droplet Digital PCR, the gene copy number can be reproducibly determined to assess the safety and stability of cell lines to ensure productivity. By distinguishing between clones containing homozygous and heterozygous edits, Droplet Digital PCR also provides a screening strategy to characterize the expected frequency of homologous recombination, further improving custom cell engineering workflows. Designing ddPCR genome edit detection assays can be easily done for any target of interest with our easy-to-use Digital PCR Assay site, allowing you to quickly identify and focus on desired clones.
bsAbs require a cost-effective, highly selective, and easy-to-scale process to achieve a suitable purity for the final product. Resin-based chromatography is often used during antibody purification workflows. Bio-Rad’s mixed mode chromatography resins have the unique ability to combine different types of interaction, such as metal affinity, ion exchange, and hydrophobic, into a single support matrix. This provides significantly improved selectivity, binding capacity, and salt tolerance to effectively purify bsAbs according to their two distinct epitope affinities while removing multiple product-related impurities to near negligible levels. Learn more about our chromatography resins for optimized large-scale bsAb purification.
Assessing Immunogenicity
The safety, efficacy, and pharmacokinetic properties of candidate bsAbs, as well as the levels of anti-drug antibodies (ADAs), need to be closely monitored throughout preclinical and clinical stages. Bioanalytical assays are essential to fully assess immunogenicity. The Bio-Rad Human Combinatorial Antibody Library (HuCAL®) platform provides rapid, reproducible, and consistently high-quality antibodies, ideal for pharmacokinetic studies and the development of immunogenicity assays. Read more about how our custom antibody service can support you.
The Future of Bispecific Therapeutics
There is an increasingly dynamic clinical and preclinical pipeline for bsAbs, exploring a range of formats, targets, and mechanisms of action. bsAbs are shaping immunotherapy treatments for both hematological and solid tumors, and effective methods to overcome challenges throughout discovery and development workflows are essential to support continued advances in the field.
Bio-Rad offers a range of tools to support scientists in their research on therapeutic candidates. To learn more about how Bio-Rad can support you in the discovery and development of bispecific antibodies, check out our antibodies page.
References
Amgen, Inc. (2021). Amgen Pipeline. amgenpipeline.com, accessed October 14, 2021.
Kamta J et al. (2017). Advancing cancer therapy with present and emerging immuno-oncology approaches. Front Oncol 7.

