As biotherapeutic modalities have diversified and production processes become more advanced, an evolution in purification strategies is also needed. Modern biotherapeutic purification must be capable of removing multiple types of impurities without compromising yield or quality, in a cost-effective manner. To meet these needs, many are turning to mixed-mode chromatography.
Biotechnology is experiencing a new era thanks to the emergence of diverse therapeutic modalities, including cell and gene therapies (for example, viral vectors, Adeno-associated viruses [AAVs], CAR T-cell therapies), monoclonal antibody (mAb) derivatives (such as Fc-fusions, bispecifics, and multispecifics), antibody-drug conjugates (ADCs), and mRNA vaccines. Furthermore, significant advancements in upstream biotherapeutic production and expression have led to an increase in titers.
Taken together, these advancements have led to the need for innovative and advanced purification solutions that are capable of effectively removing impurities — such as double-stranded DNA (dsDNA), endotoxins, host-cell protein (HCPs), and protein aggregates — while maintaining high yields. To address these challenges, mixed-mode (or multimodal) chromatography (MMC) has been receiving increasing attention as an alternative or complementary approach to traditional chromatography for both process- and lab-scale purification.
Mixed-Mode Chromatography: An Alternative to Three-Step Purification
By harnessing media capable of at least two modes of interaction, such as hydrophobic, electrostatic, hydrogen bonding, metal affinity, or size exclusion, MMC can selectively remove a broad range of impurities in a single step. This approach has been shown to improve process efficiency and productivity in comparison to a multi-step approach, without compromising biotherapeutic target recovery (Bola et al. 2023).
Bio-Rad offers several mixed-mode media combining different chromatographic elements, including hydrophobic ion-exchange ligands and hydroxyapatite media.
Nuvia cPrime Mixed-Mode Media
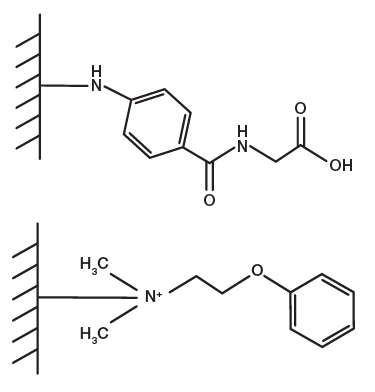
Fig. 1. Mixed-mode ligands for Nuvia cPrime (top) and aPrime 4A (bottom) Resins.
Providing highly reliable recovery across a wide range of salt concentrations and pH at high flow rates, Nuvia cPrime Media is particularly suitable for commercial manufacturing settings. Designed with a mixed-mode ligand that combines hydrophobic and cation exchange (CEX) properties (Figure 1), Nuvia cPrime Media has a large design space for binding and elution, allowing for the development of highly robust methods.
Nuvia aPrime 4A Mixed-Mode Media
Optimized to maximize mass transfer of biotherapeutics for optimal purity and recovery at high flow rates, Nuvia aPrime 4A Media is designed with both hydrophobic and anion exchange (AEX) chromatographic capabilities. Nuvia aPrime 4A Media is built upon the same rigid macroporous highly crosslinked polymer base matrix as Nuvia cPrime Media and features a strong AEX ligand together with a hydrophobic aromatic side chain (Figure 1). Example mAb purification workflows demonstrating the utility of Nuvia aPrime 4A Media are given in Figure 2.
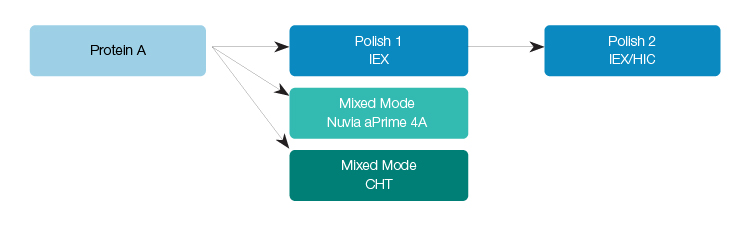
Fig. 2. mAb purification workflows using mixed-mode chromatography media to enhance the separation of mAbs in a time- and cost-efficient process. CHT, ceramic hydroxyapatite; HIC, hydrophobic interaction chromatography; IEX, ion exchange.
Ceramic Hydroxyapatite (CHT) is Mixed-Mode by Nature
The versatility of CHT Media provides researchers with multiple options to fine tune their purification workflows across a range of downstream applications for both lab- and process-scale workflows. Inherently multimodal in nature, hydroxyapatite has unique separation properties, with capabilities to separate similarly sized molecules. CHT Media is well known for its ability to remove common host cell and process-related impurities and has been used for both capture and polishing steps of challenging downstream processes for a variety of biotherapeutics, including mAbs, ADCs, nucleic acids, viruses, and more (Kallberg et al. 2012, McLaughlin 1989, Chen and Zhang 2021, and Kumar et al. 2020).
CHT Media is made up of chemically synthesized ceramic beads composed of both phosphate and calcium groups, which are sintered at high temperatures, resulting in a final product ((Ca5(PO4)3OH)2) that serves as both the support matrix and the ligand. Different sintering temperatures result in differences in pore sizes, leading to differences in binding capacities, pressure/flow characteristics, and selectivities. CHT Media facilitates metal affinity binding through its Ca2+ groups, as well as CEX interactions through its phosphate groups. Selective elution of impurities and target biomolecules is achieved by adjusting salt and/or phosphate concentrations of the mobile phase (Figure 3).
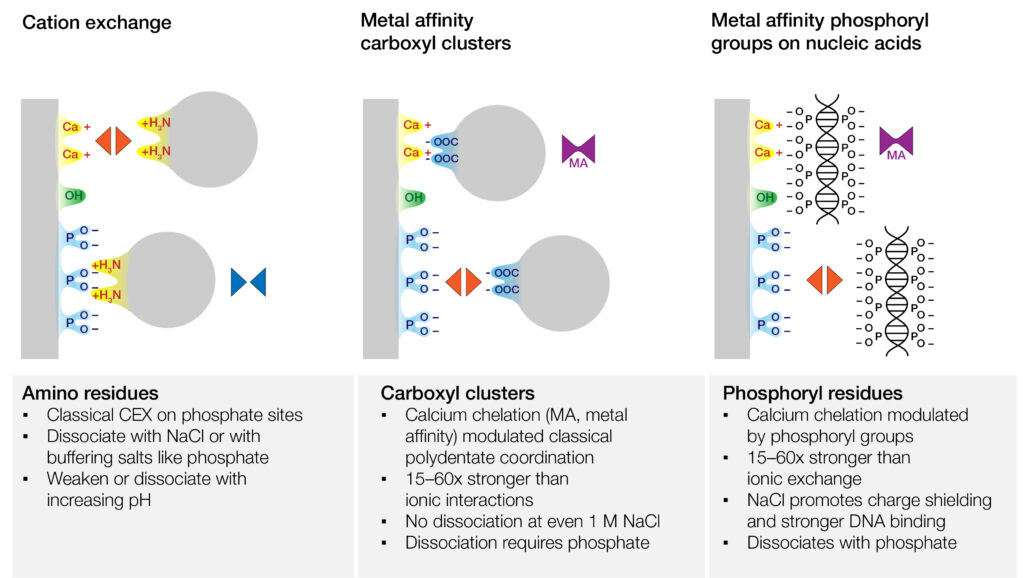
Fig. 3. Multimodal ligand chemistry of CHT Media. CEX, cation exchange; IEX, ion exchange, MA, metal affinity.
CHT Type I Media has a higher protein binding capacity than CHT Type II Media, and is well known for its successful application for purifiying acidic proteins. However, CHT Type II Media, with its larger pore size, offers better resolution for nucleic acids and certain proteins. With a very low affinity for albumin, CHT Type II Media is also well-suited for purifying immunoglobulins and large molecules such as viruses. CHT XT Media features enhanced pressure and flow capabilities and a uniform bead size along with high binding capacity, enabling a greater number of purification cycles, making it particularly ideal for large-scale manufacturing processes.

Fig. 4. CHT Media and Nuvia Resins are available in a variety of formats and volumes.
Bio-Rad offers CHT Type I and II Media in two particle sizes: 40 and 80 µm. CHT Ceramic Hydroxyapatite XT Media, the newest addition to the CHT chromatography media line, is available in a 40 µm particle size. Our CHT Media are conveniently available in a variety of prepacked formats, including RoboColumn units, resin screening plates, and Foresight Pro Chromatography Columns in sizes up to 33 cm diameter by 20 cm bed height (Figure 4). These good manufacturing practice (GMP)-ready columns are specifically designed for easy integration into bioprocessing workflows.
Incorporation of MMC into Bioprocessing Workflows
Although the current downstream manufacturing processes for traditional therapeutics, which rely on multistep monomodal purification, are well-developed, validated, and firmly established, the growing application of novel therapeutic modalities increases the demand for faster development and manufacturing. Moreover, complexities associated with the physicochemical properties of newer therapeutic modalities present unique purification challenges.
The adoption of mixed-mode media purification approaches can increase downstream process efficiency, allowing for faster and more cost-effective development and recovery. Unlike standard purification processes, MMC allows for:
- Selective single-step removal of impurities.
- Effective separation of compounds that appear homogeneous using standard purification methods.
- Establishment of optimal binding and elution conditions due to a larger design space
- Minimal feedstock processing due to high salt tolerance.
- Large capacity high-titer feed-streams (Scott 2020).
Poised to have a huge impact on high-throughput purification processes from lab-scale to manufacturing, MMC enables specifically tailored, efficient, and robust workflows across all stages of biotherapeutic development.
Visit our website to discover a wide range of mixed-mode media available in various formats.
References
Bola S et al. (2023). An alternative two-step mixed-mode approach to a monoclonal antibody (mAb) purification process. Bio-Rad Bulletin 3576.
Chen SW and Zhang W (2021). Current trends and challenges in the downstream purification of bispecific antibodies. Antib Ther 4, 73–88.
Kallberg K et al. (2012). Multimodal chromatography: An efficient tool in downstream processing of proteins. Biotechnol J 7, 1485–1495.
Kumar A et al. (2020). Design and validation of linkers for site-specific preparation of antibody-drug conjugates carrying out multiple drug copies per cysteine conjugation site. Int J Mol Sci 21, 6882.
McLaughlin LW (1989). Mixed-mode chromatography of nucleic acids. Chem Rev 89, 309–319.
Scott C (2020). Mixed-mode chromatography for purification of biopharmaceuticals (New York: BioProcess International).