Abstract
Traditional sequential chromatography typically requires intensive hands-on user manipulation, which could hinder the reproducibility of protein purification runs. Multi-D chromatography aims to improve this process while maintaining the efficacy of the traditional workflow.
Here, we compare the traditional sequential chromatography workflow to the Multi-D chromatography workflow using the NGC Chromatography System and show that both purification methods yield proteins of equivalent purity. In addition, we highlight the convenience and reproducibility of the automated NGC System.
Figures
|
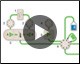
NGC Chromatography System Configuration Figure 1. Animation of the automated Multi-D process. |
Traditional Chromatography Workflow Figure 2. Traditional sequential chromatography workflow. |
|
Multi-D Chromatography Workflow Figure 3. Multi-D chromatography workflow. |
Consistency of Runs and Aggregation Determination Figure 4. Determination of aggregation. |
|
Reproducibility of Multi-D Chromatography Figure 5. Reproducibility of the Multi-D chromatography workflow |
Figure 6. SDS-PAGE comparison of the purified protein. |
Introduction
A traditional chromatography purification workflow uses independent columns run sequentially. This type of workflow consists of running a single column, collecting fractions, analyzing and pooling the fractions, preparing the chromatography system for the next run, and then repeating these steps for each additional column. This process requires significant hands-on time with considerable user involvement, requiring the user to be present throughout the duration of the workflow. This increased user intervention increases the probability of introducing errors into the workflow and potentially affects the reproducibility of each run. The labor intensive nature of the workflow also prevents the user from focusing on other tasks.
Multi-D chromatography (Figure 1) on Bio-Rad’s NGC System offers several advantages over the traditional chromatography workflow, such as convenience and reproducibility. Though this more automated purification method requires optimized single purification protocols, it allows continuous multi-step purification, providing single push-button functionality to protein purification. This not only allows the user to walk away and focus on other work, but also yields consistent and reproducible purifications.
To illustrate the value of Multi-D chromatography, we focus on a common application in the production of biologics for pharmaceutical applications — screening aggregation levels of a monoclonal antibody in tissue culture fluid. During the production of biologics, it is not uncommon to observe aggregation of the target biologic during protein expression. Aggregation often can be prevented, or at least reduced, by optimizing the media and/or growth conditions and by optimal clone selection.
Figure 1. Multi-D chromatography using the NGC System. This animation demonstrates how Multi-D automated runs hold the protein of interest until all of it has been eluted before collecting fractions. Completing the elution process by carrying out multiple runs before fraction collection speeds the purification process and the automation ensures consistency and reproducibility.
In order to optimize the production method to reduce aggregation, multiple experimental conditions must be analyzed. Typically, the monoclonal antibody is first separated from the tissue culture matrix by affinity purification on a protein A column. The sample is then analyzed by size exclusion chromatography (SEC) to resolve and quantitate the ratio of monomer to aggregate. Here, we demonstrate the advantages of the Multi-D workflow on the NGC System, enabling automated robust screening of multiple samples.
Method and Results
Sample Preparation
A purified human monoclonal antibody (mAb) immunoglobulin G (IgG) expressed in Chinese hamster ovary (CHO) cells was used to prepare all samples. To mimic samples with varying aggregation levels, the samples were incubated at low pH to induce aggregation. The mAb stock solution (10 ml, ~4.5 mg/ml) was dialyzed overnight against 500 ml 5 mM sodium phosphate, pH 7.0, at 4°C. The following day, the pH of the dialysate was adjusted by adding 50 μl 5 M sodium chloride and 100 μl 1 M glycine. The pH was further adjusted to ~pH 2.95 by slowly adding 1 M hydrochloric acid until the solution became slightly turbid. At this point, the aggregate level was monitored using size exclusion HPLC with an ENrich™ SEC 650 Column on a Shimadzu LC20A Detector.
The sample (10 μl) was injected onto the column with a mobile phase of 50 mM potassium phosphate and 100 mM potassium chloride, pH 6.8, at 1 ml/min. The monomer and aggregate were detected by absorbance at 280 nm. Once the desired ratio of aggregate (~40%) was observed, the sample was slowly adjusted back to pH 7.0 with 1 M sodium hydroxide. The sample was centrifuged at 16,000 x g for 10 min to remove the precipitate and reanalyzed by SEC to confirm the aggregate level. This stock solution was mixed with varying amounts of monomer to generate samples ranging from monomer-only (0% aggregate) to 25% aggregate. All samples were stored at 4°C prior to analysis.
Before running the samples on the NGC System, the mAb IgG mixtures were mixed with equal volumes of CHO tissue culture fluid to mimic an expressed target protein with other contaminating proteins. EDTA was added to a final concentration of 20 mM to inhibit any protease activity. For each purification, 3 ml of approximately 1 mg/ml mAb IgG in CHO tissue culture fluid was injected onto the column (~3 mg mAb IgG).
NGC System Configuration
An NGC™ Discover 10 Pro Chromatography System with an additional column switching valve was used for this purification. The Figure 1 animation demonstrates the automated Multi-D process.
A
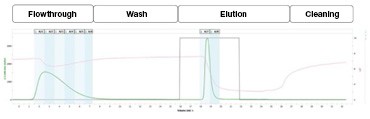
B

Figure 2. Traditional sequential chromatography workflow. The sample was manually injected onto the first column (protein A affinity) and fractions were manually neutralized and pooled. The chromatogram illustrates the various phases of the purification (A). Part of the eluate from the column was then manually injected onto the second column (SEC) and the fractions were collected. The SEC chromatogram illustrates the ability to resolve the monomer from the aggregate (B). The blue vertical bands represent the collected fractions.
Chromatography Columns and Buffers
For both purification workflows, a 1 ml Bio-Scale™ Mini UNOsphere SUPrA™ Affinity Column (protein A) and a 10 x 300 mm ENrich SEC 650 Column were used. In addition, a 10 ml Bio-Scale™ Mini Bio-Gel® P-6 Desalting Column was used for the Multi-D purification to buffer exchange the eluate from the affinity column. The load and wash buffers for the three columns were 10 mM sodium phosphate, pH 7.8, and 150 mM sodium chloride with 10 mM EDTA, respectively. The elution buffer for the affinity column was 100 mM sodium citrate, pH 3.0.
Traditional Sequential Chromatography Workflow
In this workflow (Figure 2), the mAb IgG sample was manually loaded into a 5 ml sample loop and injected onto the affinity column at a flow rate of 1.5 ml/min. The mAb IgG was step-eluted from the column and each 1 ml fraction was neutralized with 100 μl 1 M Tris, pH 8.0 (A). The fractions containing the mAb IgG were then pooled and 0.7 ml was manually loaded into a sample loop and injected onto the SEC column for aggregate analysis (B). To reanalyze the samples and to gauge the reproducibility of the system, this entire workflow was repeated in triplicate for each sample. Representative chromatograms of the purification are shown in Figure 2. By integrating the peaks in the chromatogram, the percent recovery (area of elution peaks/sum of the area of the flowthrough and elution peaks) was calculated to be 30.9 ± 0.1% (n = 3).
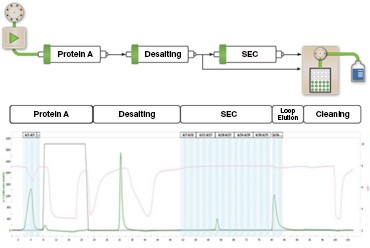
Figure 3. Multi-D chromatography workflow. The samples were loaded onto the column by the sample pump and the sample was purified on a protein A column and then buffer exchanged on the desalting column. Part of the sample was then injected onto the SEC column for aggregate analysis. Following this, the remaining sample, stored in the sample loop, was collected. The chromatogram shows the phases of the purification. The blue vertical bands represent the collected fractions.
Multi-D Chromatography Workflow
In this workflow (Figure 3), the NGC System automatically loaded the sample, purified and analyzed the sample for aggregates, and collected the eluted protein fractions from the SEC column for each sample. Samples with varying levels of aggregation were plumbed into separate ports of the sample inlet valve. Once the method was started, the sample pump pulled a sample from the specified port into the sample pump through the injection port and onto the affinity column at a flow rate of 1.5 ml/min. The sample was eluted off the affinity column directly onto the desalting column to buffer exchange the protein from a low pH elution buffer to a neutral pH buffer. The sample was then circulated through the detector and returned through the outlet valve and sample injection module to the sample loop. A representative chromatogram of the purification is shown in Figure 3. The percent recovery was calculated to be 31.8 ± 0.1% (n = 3), which demonstrates that the different purification workflows are comparable.
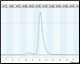
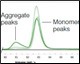
A small fraction of the sample (0.7 ml) was injected from the sample loop onto the SEC column for aggregate analysis. Figure 4 illustrates: (a) the consistency between runs with samples of varying levels of aggregation (0%, 10%, and 25%); (b) the separation of the monomer from the aggregate; and (c) the fact that the various aggregation levels can be clearly differentiated.
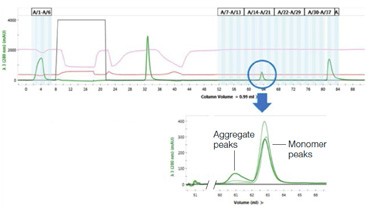
Figure 4. Determination of aggregation. The chromatogram depicts the overlay of three different samples on the SEC column containing varying amounts of aggregation (0%, 10%, and 25%). The highlighted peaks represent the degree of aggregation in the different samples.After SEC analysis, the remaining protein in the sample loop was sent to the fraction collector. The columns were then automatically cleaned and the next sample loaded to repeat the analysis.
The reproducibility of repeat sample injections is highlighted in Figure 5 with an overlay of the tandem chromatograms for the purification of a sample with 0% aggregation.
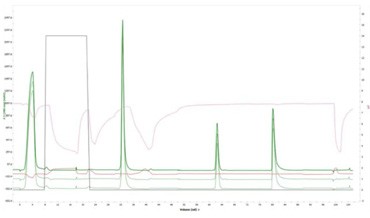
Figure 5. Reproducibility of the Multi-D chromatography workflow. This figure shows three repeat injections of the same sample (monomer with 0% aggregation) using the sample pump.
SDS-PAGE Analysis
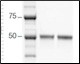
To compare the final purity, the product of each purification scheme was analyzed by SDS-PAGE using a 4–15% Criterion™ TGX Stain-Free™ Gel (Figure 6). The eluate from the traditional purification was diluted 1:20 in 1x Laemmli Sample Buffer and the eluate from the Multi-D purification was diluted 1:3 in 1x Laemmli Sample Buffer. Precision Plus Protein™ Unstained Standards were used to determine the molecular weight of the samples. An equal volume of sample (20 μl) was loaded and the gel was visualized using the ChemiDoc™ MP System with stain-free imaging. The gel was activated for 2.5 min prior to image acquisition for 2 sec.
As shown in Figure 6, the proteins purified by both workflows showed equivalent purity, demonstrating that Multi-D chromatography delivers the same quality results as traditional sequential chromatography.
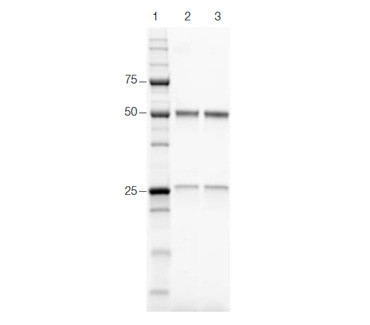
Figure 6. SDS-PAGE comparison of the purified protein. The purity of the purified proteins using both workflows was compared by SDS-PAGE and the proteins were visualized using stain-free imaging. Lane 1, Precision Plus Protein Unstained Standards; lane 2, traditional sequential chromatography eluate; lane 3, Multi-D chromatography eluate.
Conclusions
- The recovery and purity of biologics samples using both purification methods are equivalent
- The Multi-D workflow provides one-push functionality that enables the user to start a method and walk away
- Using this automated purification method, the varying levels of aggregation can clearly be distinguished and quantified, ensuring an optimized biologics production method
- The automated Multi-D workflow is highly reproducible, providing confidence that the purified sample will meet the user’s rigorous demands, run after run
Advantages of Using the NGC Chromatography System
Developing a Multi-D method is not as straightforward as developing a traditional sequential one. To ease the adoption of more automated chromatography, the NGC System has been designed to provide a single laboratory chromatography solution that scales to fit the user’s throughput requirements. First, all NGC Systems come with ChromLab™ Software, which provides a simplified approach to programming Multi-D chromatography methods with minimal expertise. Pre-written templates included in the software provide model methods that can be used directly or adapted with minor changes to either phase parameters or individual method steps depending on the user’s requirements. ChromLab Software has both phase and step libraries that can be used to quickly build novel methods or customize an existing template. In addition, the scouting feature within the software helps determine the optimal purification conditions in an automated, systematic manner for each individual purification step prior to transitioning the conditions into a Multi-D method. The software’s streamlined data processing allows easy analysis across multiple purification runs and provides a variety of viewing and data analysis options. With its intuitive graphical interface and convenient touch-screen control, the software controls all functions of laboratory-scale protein purification, including instrument setup and calibration, method development, real-time monitoring and system control, and chromatogram comparison.
Next, the NGC System is modular, easily allowing the addition or exchange of modules to meet the user’s requirements. This makes the system extremely versatile and supports multiple purification schemes. With two buffer inlet valves, the NGC System can accommodate different buffers, allowing the user to couple columns with different buffer requirements and support multiple steps including cleaning and storage. Using a single sample inlet, up to eight different samples can be analyzed with the push of a single button. The modularity of the system also allows increased throughput with the addition of a second sample inlet or of the NGC Autosampler to enable the analysis of as many as 96 samples. The NGC System can accommodate up to three column switching valves supporting up to 15 different columns. This provides independent pressure monitoring and reverse elution capability for each column. Further, depending on the configuration, the system
provides the option of having a single sample loop per sample or a single column per sample if desired. Moreover, outlet valves allow additional control over sample collection. Finally, the fact that the entire process can be automated allows the purification and analysis to be run with minimal user intervention, freeing up the user and maximizing efficiency.
To provide support when developing Multi-D chromatography methods on the NGC System, Bio-Rad has developed a helpful how-to guide — NGC Chromatography Systems Tandem/Multistep Plumbing Guide (bulletin 6674) — which can be accessed by clicking the link or by visiting bio-rad.com and searching for the bulletin number.
Shimadzu is a trademark of Shimadzu Corporation.
Precision Plus Protein Standards are sold under license from Life Technologies Corporation, Carlsbad, CA for use only by the buyer of the product. The buyer is not authorized to sell or resell this product or its components.