Dr. Christopher Fraser and the members of his lab in the Department of Molecular and Cellular Biology at the University of California, Davis, want to better understand the fundamental cellular process of protein synthesis. The genomic DNA in each cell of an organism encodes the same set of genes, but gene expression is regulated so that only certain proteins are produced at appropriate times in each cell, resulting in diverse cellular functions. The control of the first step of protein synthesis is the regulation of DNA transcription into mRNA. The next step, the translation of mRNA into protein by the ribosome, is also tightly controlled in normal cells, and dysfunctional regulation is responsible for disorders such as autism, Type I diabetes, and cancer.

Assistant Professor, Molecular and Cellular Biology
University of California, Davis
“If we can reveal the code hidden in the mRNA structure and how the translation machinery is regulated by it,” Fraser says, “perhaps we can understand how to manipulate the process so that we can correct it when it goes wrong.”
Addressing Fundamental Biological Questions
Protein translation is one of the most essential yet least understood basic cellular processes due to its complexity. The members of the Fraser lab study the control of protein translation at a very basic level. Fraser explains the importance of such fundamental research:
“Because of the need for tight control of the protein synthesis machinery, it’s critical that we understand exactly how the translation machinery works,” he says. His studies build on a large body of prior research. “A lot of work has been done over the past 40 years to catalog all the components of the system toward understanding the underlying mechanism and its regulation,” he says. The Fraser group reassembles these components in vitro to learn how they interact.
The ribosome is a large RNA-protein complex arranged into two subunits. The smaller (40S) subunit binds the mRNA transcript, and the larger (60S) subunit binds transfer RNA (tRNA) bearing amino acids that are joined to form proteins. The 40S subunit interacts with a number of protein complexes called initiation factors, each composed of smaller subunits (Figure 1A). The diverse components of the cellular translation nanomachinery combine, dissociate, and change conformation in a complicated sequence during the initiation and completion of protein translation. Fraser and his coworkers study the thermodynamics and kinetics of this intricate biomolecular dance (Fraser 2009).
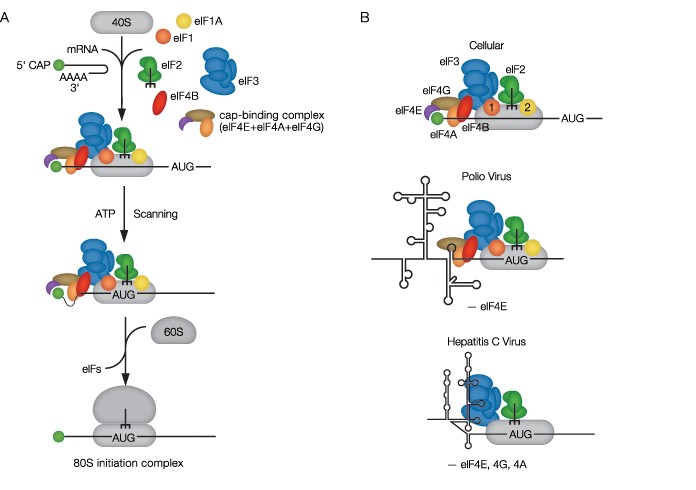
Fig. 1. A, the assembly of the ribosomal complex in eukaryotic translation initiation; B, cellular and viral conformations of mRNAs bound to ribosomal complexes during translation initiation. AUG indicates the translation start site.

Prof. Chris Fraser, a researcher at UC Davis, and graduate student Nancy Villa using the NGC system. The system has increased the Fraser Lab’s chromatography throughput by 25% while accommodating multiple purification schemes by multiple users.
Exploring the Intricate Mechanisms of Protein Translation
Fraser became fascinated with trying to understand the mechanism by which mRNA is translated into protein in a project studying human protein synthesis during his undergraduate studies in the UK. After completing his PhD at Sussex University, Fraser conducted postdoctoral research on reconstituting human protein synthesis in vitro using purified components in John Hershey’s lab at UC Davis. After another postdoctoral stint at UC Berkeley in Jennifer Doudna’s lab, where he acquired a lot of experience working with RNA, he became particularly interested in trying to understand the differences in how cellular mRNAs and viral mRNAs are translated, and this has become the focus of his own lab at UC Davis, a university hosting both basic and applied research. He notes, “This setting allows researchers from many different areas of expertise to interact. Few institutions have a medical school, a veterinary school, and an agricultural school. Here, I feel that my research can move in any direction using any model organism that I wish.”
Fraser elaborates: “For cellular mRNAs, we are trying to understand why some mRNAs are translated more efficiently than others. This regulation appears to involve a region of the mRNA that does not encode amino acids (the five-prime untranslated regions, or 5′ UTRs) and is likely connected to the amount of structure that the RNA can adopt,” depicted as stem-and-loop, or “hairpin,” structures in Figure 1A. “More complex structure generally results in less protein being made because the RNA structure must be melted [the twinned strands separated] so that the ribosome can engage the mRNA and locate the translation start site.”
The relationship between mRNA structure and translation levels constitutes a long-standing mystery in this field. The Fraser lab focuses on how structures in the 5′ UTRs of human mRNAs control translation efficiency. Fraser notes, “This is important because proliferative and prosurvival mRNAs typically possess high degrees of structure in their 5′ UTRs. These structures are generally thought to reduce translation efficiency but somehow become preferentially translated under growth-promoting conditions such as cancer. Our research aims to reveal the mechanism by which eukaryotic initiation factors restructure 5′ UTRs so that the ribosome can bind and initiate efficiently. We study this process using a reconstituted system to accurately monitor these events in real time.”
A milestone in this work was the establishment of a dual role for the eukaryotic initiation factor (eIF) 4E complex, previously known to recruit mRNA to the 40S subunit by binding to the 5′ cap structure found on eukaryotic mRNA. The eIF4E protein also functions as an activator of eIF4A, a helicase that unwinds helical double-stranded mRNA duplexes into single-stranded mRNA that can then be translated by the ribosome. A crucial experimental technique in the elucidation of this activity was pioneered in the Fraser lab: a fluorescent helicase assay that enables real-time monitoring of eIF-mediated mRNA restructuring (Ozes et al. 2011).
Demanding Protein Purification Routines
“We have taken a biochemistry approach to deciphering the mechanism of translation,” Fraser explains. “We essentially purify all of the components needed to assemble the machinery in vitro and then combine them in different combinations and amounts to see how well the machinery works. By understanding the mechanism in this way, we hope to determine how defects in the machinery can be corrected in disease states.”
The reconstruction of the ribosomal complex presents particular purification difficulties due to the widely varying properties of the components, such as molecular sizes ranging from a few kilodaltons to over one megadalton. The simpler translation initiation factors are produced as tagged recombinant proteins in transgenic bacterial or insect cells, allowing for a relatively streamlined purification protocol. Larger and more complex factors are obtained by fractionating endogenous proteins from mammalian cells, a process that involves separating these relatively rare native complexes away from a high background of total cellular proteins while preserving their structures and activity.
“Our research relies heavily on purifying protein components that often require at least two or three columns. With multiple researchers in the lab, we need to maximize our throughput,” Fraser says. “We routinely purify ~10 different protein factors needed to reconstitute our in vitro system, so it’s important that it is easy to switch between protocols and columns.”
As limitations in protein purification represent a bottleneck in the experimental workflow in the lab, Fraser was motivated to seek an alternative to their current fast protein liquid chromatography (FPLC) system in hopes of reducing the amount of time and effort spent on this stage of the bench work. The Fraser lab was therefore an ideal setting for the beta testing of Bio-Rad’s NGC chromatography system. In collaboration with the product development team at Bio-Rad, Fraser and his team of researchers put the new NGC unit through a regimen of testing over several months of regular use in the lab, providing Bio-Rad engineers with valuable feedback in the process.
In comparison with their previous FPLC system, Fraser states “We found the NGC system to be extremely intuitive to use – more like an Apple product vs. our older PC-like system. Programming and general system control is very simple. The front-facing interface allows for easy switching between columns without reaching around to the side of the machine. Pump washes are particularly quick and easy to achieve between buffer changes.”
The NGC Chromatography System Enables the Fraser Lab to Maximize Protein Purification Throughput
Traditional preparative chromatography systems tend to be highly complex in terms of connectivity and operation, which can be intimidating to new users, such as new graduate students or undergraduate research assistants. “Training junior researchers how to use the previous system often required multiple runs before they were comfortable, which is dramatically reduced — to only one or two runs — using the NGC system,” notes Fraser, adding “The sample pump was particularly useful to us since it avoids the need for traditional superloops. The removable modules are also a nice feature so that it will be possible to minimize downtime in case of problems.” As the workflow in the Fraser lab involves diverse purification needs, the group also appreciated the flexibility of the modular configuration of the NGC system, which allows researchers to purchase a basic system and then reconfigure or expand with additional modules as the lab explores new research threads.
Many of the proteins and protein complexes studied in the Fraser lab are temperature sensitive. Older, bulkier chromatography systems often had to be kept in dedicated walk-in coolers to maintain the samples at 4ºC, forcing researchers to spend hours in the cold room. The Fraser lab set up the NGC system inside a deli fridge, in which it was purposely designed to fit. It can be operated remotely via personal computer or by a detachable touch-screen controller that sits directly outside the fridge, providing ready access to the onboard software. Other aspects of the NGC system that were well liked by Fraser lab members were the ease of programming, using the drag-and-drop method creation procedure, and the direct visualization of the sample flow path, highlighted in green on the system setup screen. After configuring the system in the software, connecting the various hardware components, such as pumps, mixers, and detectors, is greatly simplified by the Point-to-Plumb™ feature.
Yield and purity considerations are paramount with product scales of micrograms to milligrams. Fraser notes that the ability to quantify individual proteins as they come off the column was a very useful feature of the NGC system for him. “Knowing how much protein we have and in what purity is essential to subsequent phases of our experiments,” he says. Fraser also related his positive experience as a beta-tester of the NGC system, saying “Bio-Rad asked for our input; they listened to us and incorporated the features we said we wanted. As a result, we ended up with a system that really does what we want it to do,” adding “Customer support has been excellent.” Due to his active involvement in the development of the NGC system in collaboration with Bio-Rad, Fraser says that he feels fully confident in the superiority of the system: “I’d recommend the NGC system to any researchers whose work involves routine or difficult protein purifications.”
The Bottom Line: Increased Research Productivity
When asked what having the NGC system in his lab will enable his group to do in the future, Fraser summarizes it thus: “Throughput and reliability are extremely important in a lab with many researchers using the same instrument. The NGC system will boost our capacity to purify multiple proteins more quickly. We used to do four runs a day, now we can do five — perhaps a 25% increase in throughput — because it is much quicker to switch between users.” This enhanced capacity promises to relieve a major bottleneck to research productivity in many labs, a sentiment echoed by senior proteomics researchers, who have often cited chronic problems with chromatography systems as a major source of downtime.
Although research at a basic level such as that conducted in the Fraser lab is typically far removed from technological applications, when asked to speculate on the scope of possible uses for the knowledge obtained in their work, Fraser replies that the study of translation mechanisms could have numerous therapeutic uses. For example, “Understanding how viruses hijack ribosomes to translate their mRNA may lead to the development of drugs that can specifically inhibit viral translation (Fraser and Doudna, 2007). Our studies could also help elucidate the role of translational regulation in cancer.” In a world living with an HIV epidemic with no cure in sight and facing increasing cancer mortality, such technologies could be of great benefit to people all across the globe.
References
Fraser CS (2009). The Molecular Basis of Translational Control. In Hershey, JWB, Ed., Progress in Molecular Biology and Translational Science, Vol. 90, pp. 1–51.
Fraser CS and Doudna JA (2007). Structural and mechanistic insights into hepatitis C viral translation initiation. Nat Rev Microbiol 5(1):29–38.
Ozes AR et al. (2011). Duplex unwinding and ATPase activities of the DEAD-box helicase eIF4A are coupled by eIF4G and eIF4B. J Mol Biol, 412, 674–687.

