Abstract
Inflammation is the leading cause of mortality worldwide and is indicated in the following eight major disease areas: autoimmune diseases, cancer, cardiovascular diseases, infectious diseases, diabetic complications, metabolic disorder complications, neurological diseases, and pulmonary diseases. Magnetic bead–based assays are widely used for the quantitation of relevant biomarkers implicated in inflammation. Bio-Rad’s Bio-Plex Pro Human Inflammation Assay measures 37 of the most relevant inflammation biomarkers, including TNF superfamily proteins, Treg cytokines, IFN proteins, and matrix metalloproteinases, in a single multiplex panel. This report provides the performance characteristics of the Bio-Plex Pro Human Inflammation Assay Panel, including assay working range, sensitivity, precision, and linearity of dilution, and illustrates the validation data generated using this panel.
Figures
|
Assay Performance Characteristics Table 1 |
Fig. 1. Standard curves with assay controls and serum samples. |
|
Accuracy of Bio-Plex Pro Human Inflammation Assays Fig. 2. Linearity of dilution. |
Fig. 3. Serum levels of biomarkers in normal and disease groups. |
|
More Reliable Sample Detection with the Bio-Plex Pro Human Inflammation Assays Fig. 4. Comparison of the Bio-Plex Pro Human Inflammation Assays to an equivalent multiplex assay from another vendor. |
Rigorous Assay Validation
All Bio-Plex Pro Assays undergo a rigorous evaluation that includes the following parameters. Of these, the ones highlighted in bold are indicative of assay performance (Table 1):
- Specificity (cross-reactivity)
- Accuracy (recovery) in key sample matrices; defined as the percentage of the observed concentration relative to the expected concentration of a known amount of analyte within the assay working range
- Inter- and intra-assay precision; defined as the coefficient of variation (%CV) at concentrations within the assay working range
- Sensitivity (limit of detection, LOD); defined as the concentration of analyte for which the fluorescence intensity signal is two standard deviations above the background signal
- Assay working range; defined as the range of concentrations within which the assay is precise and accurate. Boundaries of the assay working range are defined by the lower limit of quantification (LLOQ) and the upper limit of quantification (ULOQ)
- Linearity of dilution
- Parallelism and matrix effect
- Performance characteristics in real samples
Table 1. Representative performance characteristics.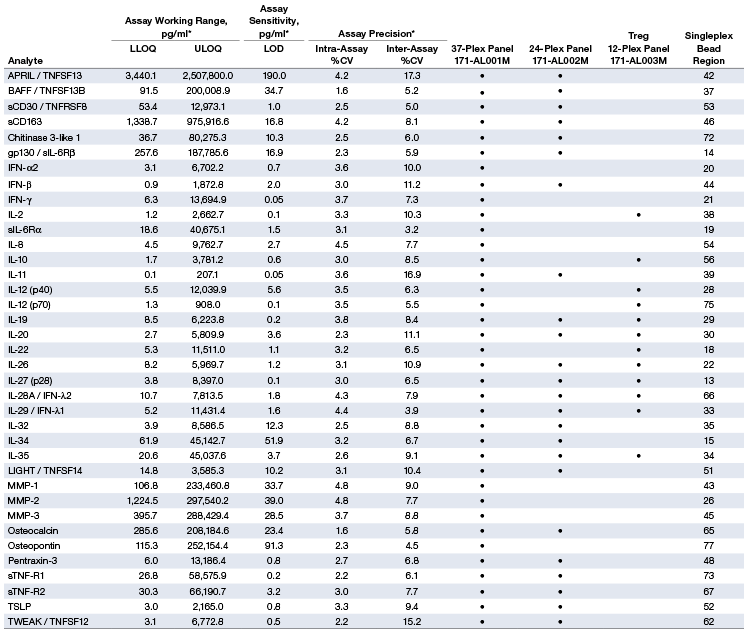
* The LLOQ, ULOQ, LOD, and inter-assay precision %CV are mean data determined from three independent multiplex assays in a serum-based matrix. Intra-assay %CV is derived from one representative assay. LLOQ and ULOQ are defined as the boundary standard curve points in which the performance specifications of individual standard points were met for a 10% intra-assay CV and recovery range of 70–130%. Data were generated using the magnetic workflow with the Bio-Plex Pro Wash Station.
Bio-Plex Pro Assay Working Range
The assay working range should encompass the biological range of expression in order to be useful in research. Bio-Plex Pro Assays are developed and optimized to ensure real sample data fall within the quantifiable regions of the assay as demonstrated by comparing the standard curves of assay controls to biological samples (Figure 1).
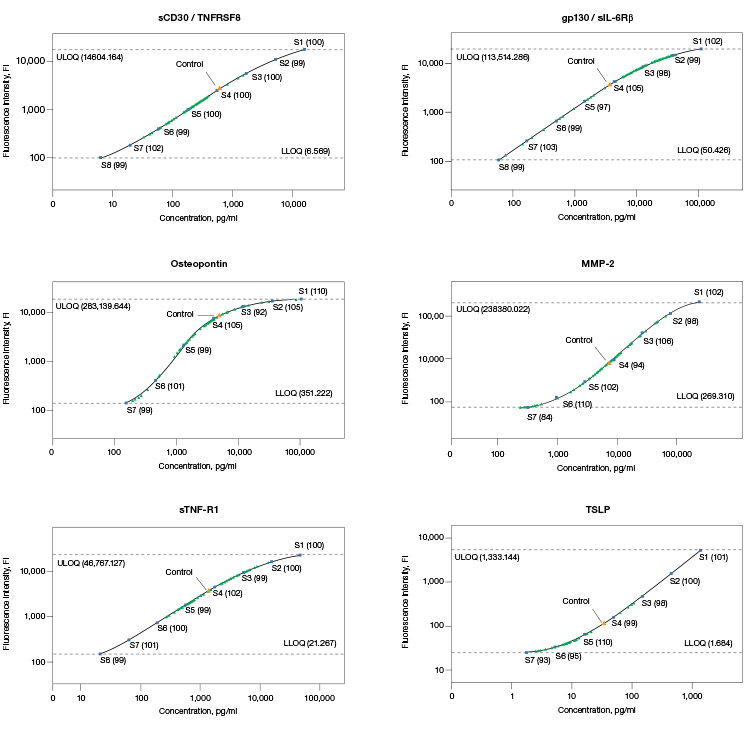
Fig. 1. Standard curves with assay controls and serum samples. Standard points were prepared by serially diluting a reconstituted standard fourfold to generate an eight-point standard curve. Standard points with % recovery (■); samples (▲). Data were generated in Bio-Plex Manager™ Software.
Accuracy of Bio-Plex Pro Human Inflammation Assays
Linearity of dilution displays the ability of an assay to generate measured values from complex samples by comparing the accuracy over a range of sample dilutions. Bio-Plex Pro Assays are designed and validated with high linearity to return accurate results from complex matrices (Figure 2).
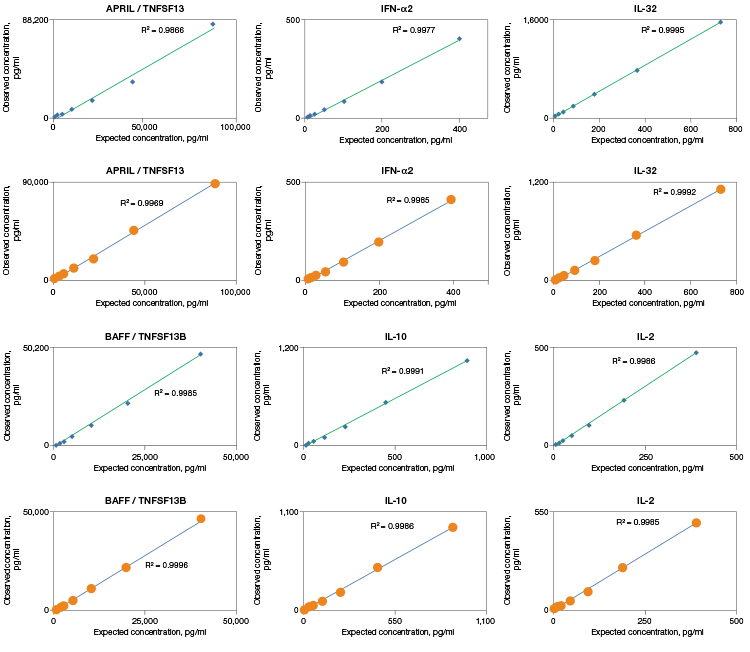
Fig. 2. Linearity of dilution determines the suitability of a standard curve for reflecting relative quantities of an analyte in a complex matrix. Linearity of dilution was assessed by spiking a known quantity of recombinant antigen into human serum and plasma matrices. Observed and expected analyte concentrations were plotted and the correlation coefficient (R2) values reflect linearity in signal response. Serum (♦); plasma (●).
Detection of Analytes
Bio-Plex Pro Assays are tested with samples from multiple sources to ensure target analytes are detected within normal biological expression levels and levels associated with disease (Figure 3).
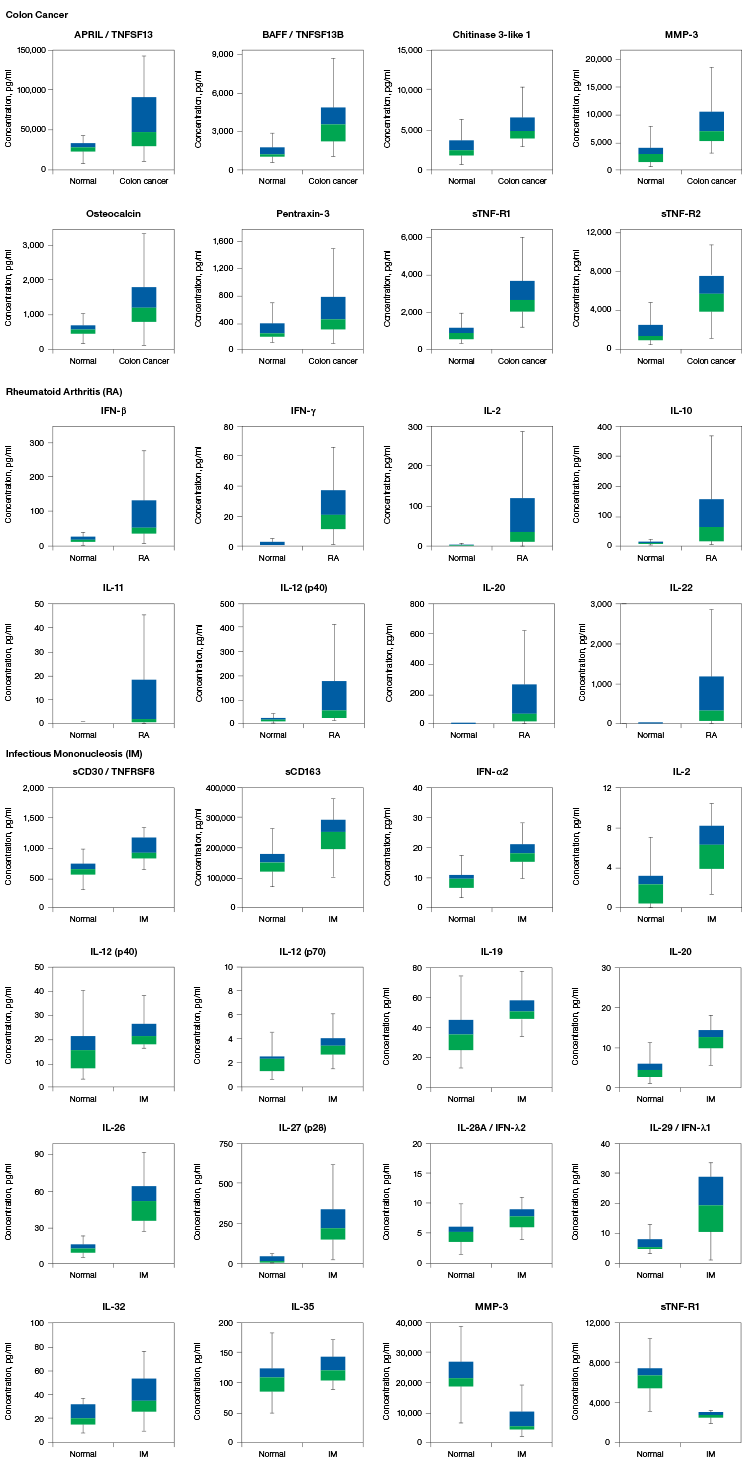
Fig. 3. Box-and-whisker plots showing serum levels of biomarkers in normal and disease groups. Colon cancer: normal serum n = 26, disease serum n = 17; rheumatoid arthritis (RA): normal serum n = 19, disease serum n = 120; infectious mononucleosis (IM): normal serum n = 19, disease serum n = 16. Please refer to the key on the left to interpret the plots.
Bio-Plex Pro Assays Provide More Reliable Sample Detection
The Bio-Plex Pro Human Inflammation Assays were compared to overlapping assays from vendor R to highlight more reliable sample detection in serum (Figure 4).
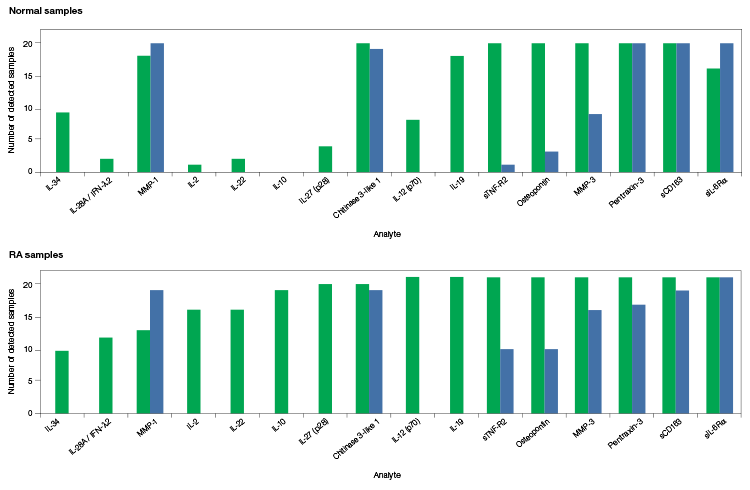
Fig. 4. Comparison of the Bio-Plex Pro Human Inflammation Assays to an equivalent multiplex assay from vendor R. Serum samples from 20 normal donors (top panel) and 21 donors with severe RA (bottom panel) were collected and evaluated for the presence of various analytes. The number of samples that the analytes were detected in is shown. The Bio-Plex Pro Human Inflammation Assays detected target proteins in 67% more samples than assays from vendor R. Vendor R’s 21-plex assay required two base kits and separate runs to accommodate the different sample dilution requirements for certain analytes, while the Bio-Plex Pro 37-Plex Assay required only a single run and single dilution. Bio-Plex Pro Assays (■); vendor R assays (■).
Product Configuration Options
Table 2. Comparison of features for each ordering option. 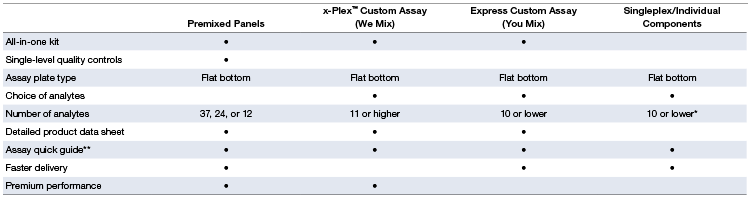
* Due to the 10x concentration of detection antibodies in singleplex sets, a maximum of ten individual sets can be mixed by the end user.
** Visit bio-rad.com/bio-plex for assay quick guides and manuals.