Although chromatography resins are usually thought of as tools to purify medicines, groundbreaking treatments now utilize resin beads as an integral component of the therapy itself. These therapies are essential for treating certain cancers and inflammatory disorders.
Chromatography resins are typically used to purify and analyze biomolecules for therapeutic applications, owing to their capacity to produce high-yield, high-purity end products (Sánchez-Trasviña et al. 2021). Recently, beyond their role in analytical and manufacturing processes, it has been discovered that the unique properties of resins also support their use in therapies delivered directly to patients. Ion exchange resins, for example, have numerous pharmaceutical applications in both controlled and site-specific drug delivery, as electrostatic interactions allow for precise control of drug loading and release (Kamble Ravindra et al. 2014).
Resins have commonly been used as the basis of microspheres — small spheres composed of biocompatible materials ranging from 1 to 1,000 µm (Sinha et al. 2004). These microspheres can be conjugated to various therapeutic agents, such as small molecules, proteins, nucleic acids, and radionuclides, and administered either locally or to a target area through intravenous injection (Häfeli 2001; Kim and Pack 2006). The biodistribution of microspheres is highly dependent on the physicochemical properties of the matrix substance.
Bio-Rad offers a wide range of chromatography resins, featuring diverse base beads and ligand chemistries, including ion exchange resins. Among them, Bio-Rad Aminex cation exchange resins — composed of a styrene-divinylbenzene copolymer with attached sulfonic acid functional groups — are strong cation exchangers. These resins have been incorporated into microsphere-based therapies for clinical use (Table 1). Additionally, Bio-Rad Bio-Rex and AG Resins, which offer either weak cation or weak anion exchanger properties, have also been used in therapeutic applications. Here, we describe how Bio-Rad resins provide a robust foundation for therapeutic purposes and review their clinical applications through current examples from the literature.
Table 1. Bio-Rad ion exchange chromatography resins used as components of therapies delivered directly to patients.
|
Type of Ion Exchanger |
Cation Exchangers |
Anion Exchangers | ||
| Resin | AG 50W | Aminex 50W-X4 | Bio-Rex 70 | Bio-Rex 5 |
| Exchanger strength | Strong | Strong | Weak | Weak anion |
| Functional group | Sulfonic acid (-SO3–) | Sulfonic acid (-SO3–) | Carboxylic acid (-COO–) | Tertiary and quaternary amines (R-N+(CH3)2C2H4OH) |
| Base matrix composition | Styrene-divinylbenzene | Styrene-divinylbenzene | Macroreticular acrylic polymer lattice | Styrene-divinylbenzene |
| Cross linkage, % | 2–12 | 4 | N/A | 4 |
| Particle size, µm | 53–106, 63–150, 75–180, 106–300, 180–425, 300–1,180 | 25–37 | 45–75, 75–150, 150–300, and 300–1,180 | 45–75 and 75–150 |
| pH stability | 0–14 | 0–14 | 5–14 | 0–14 |
| Ionic form | Hydrogen | Hydrogen | Sodium | Chloride |
Favorable Characteristics of Chromatography Resins as Carriers of Therapeutic Agents
Resins themselves are inert carrier molecules. However, their inherent physicochemical properties, including size, shape, and surface charge, along with pathophysiological factors (for example, the tumor microenvironment) are also leveraged to achieve site-specific targeting (Bazak et al. 2014). For instance, resin particles preferentially accumulate at neoplastic tissues, such as tumors, due to leaky vasculature and impaired lymphatic drainage. Particle size determines whether a resin will travel to the capillary bed, whereas its charge may affect its circulation time in the bloodstream (Häfeli 2001).
Resin-based microspheres have a density comparable to that of blood (d’Abadie et al. 2021), which may influence their suspension in the arterial flow and final tissue distribution. In a surrogate hepatic arterial system, resin-based microspheres demonstrated significantly greater penetration depths than higher-density glass microspheres (Jernigan et al. 2015). Other chemical properties can also impact particle behavior in circulation. For example, SIR-Spheres — microspheres manufactured with divinylbenzene copolymer resin (Aminex 50W-X4) labeled with yttrium-90 (90Y; Sirtex and Gray 2002) — are non-thrombogenic due to surface sulfonation. These Aminex-based SIR-Spheres have been effective agents for selective internal radiation therapy (SIRT) (Figure 1).
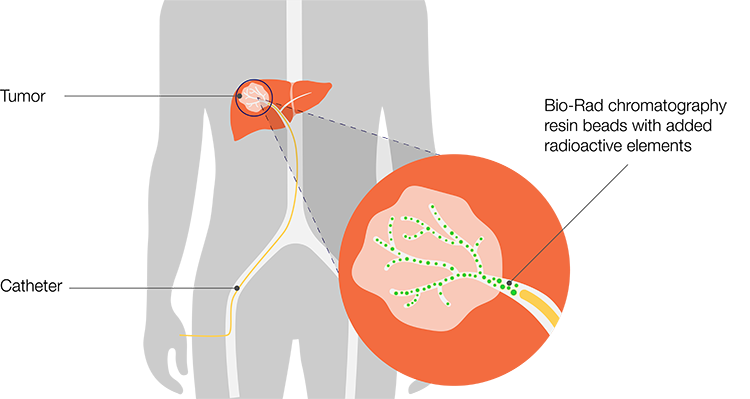
Figure 1. Selective internal radiation therapy (SIRT). Treatment involves threading a catheter to the target site, through which therapeutic microspheres are directed and delivered.
Resin stability also affects the loading of therapeutic agents, such as radionuclides, as some resins lack the capacity and/or binding affinity to be efficiently labeled with certain radioisotopes (Häfeli 2001). 90Y in its ionic form can be taken up by bone marrow, leading to severe toxicity. Thus, 90Y must remain bound to its carrier in vivo. Among various cation exchange resins, Bio-Rex 70 — an acrylic polymer with carboxylic functional groups — demonstrated superior stability for 90Y intraarterial radioembolization in an animal tumor model (Schubiger et al. 1991). Bio-Rex 70, Bio-Rex 5, and AG-50W Resins have also been labeled with holmium-166 (166Ho), resulting in high yields (>94–99%) and >95% stability in saline and serum in vitro (Subramanian et al. 2018). Further, 166Ho-labeled microspheres showed in vivo stability and target site retention in an animal model.
Therapeutic Applications of Chromatography Resins

Bio-Rad offers a complete line of chromatography products including innovative chromatography resins and media in formats for screening, laboratory, and biomanufacturing use.
Transarterial Chemoembolization and Radioembolization (TACE/TARE)
Targeted delivery of anti-cancer therapy remains a major goal as systemic treatments carry the risks of poor selectivity for tumor cells and toxic effects on healthy tissue (Welling et al. 2023). Liver cancers, including primary or secondary tumors such as hepatocellular carcinoma (HCC) and metastatic colorectal cancer (mCRC), exhibit altered vascularization, where oxygen is received from the hepatic artery (in contrast to normal liver tissue, which receives oxygen from the portal vein). By exploiting this altered vascularization for targeted delivery, microspheres administered via the transarterial route can become lodged in vasculature surrounding the tumor, where they release encapsulated chemotherapeutic drugs or deliver stable isotope-labeled particles for concentrated doses of ionizing radiation. In a prospective, multicenter, observational cohort of 498 participants with mCRC treated with transarterial radioembolization (TARE) using 90Y-labeled microspheres, the median overall survival (OS) was 15.0 months (Emmons et al. 2022). In contrast, chemorefractory mCRC typically has a median OS of 4–5 months.
Gene Therapy
Adenoviral vectors show potential for gene therapy, but their clinical application is limited due to stimulation of the immune response and issues with tissue targeting. However, attaching adenoviral vectors to cationic liposomes or polymers has been shown to mitigate immunogenicity and improve gene delivery (Natsume et al. 2000; Worgall et al. 2000). In an in vitro feasibility study, Aminex 50W-X4 Resin was used as a carrier for liposomal-adenoviral conjugates (Steel et al. 2004). Adenovirus-liposome complexes were efficiently bound by microspheres, with 98% of the available liposome and adenovirus incorporated. These complexes demonstrated sustained release onto a cell monolayer over 24 hours. Using microspheres as a carrier reduced the toxicity of adenovirus-liposome complexes, highlighting their ability to achieve targeted treatment.
Additional Considerations
Beyond optimizing physicochemical properties to achieve specific therapeutic goals, several additional factors must be considered when selecting chromatography resins for clinical or biotherapeutic use. These include sourcing products that meet stringent standards (such as ISO 13485:2016 for medical devices) from manufacturers committed to quality and effective quality management systems. The supplier’s market history and successful industry partnerships should also be evaluated. Additional considerations include method and process development support services, scale-up, transfer, and regulatory compliance. Lastly, to meet supply needs and avoid disruptions, a reliable worldwide distribution network that ensures the security of supply and adheres to sustainable manufacturing and business practices is essential.
Conclusions
Chromatography resins allow for efficient and precise control over the incorporation of drugs or radionuclides, as well as target site delivery and retention. The physicochemical properties of Bio-Rad resins, such as Aminex, AG, and Bio-Rex Resins, enable sustained drug release or concentration of radioactive particles at the site of interest with minimal toxicity to surrounding tissues. Preclinical studies in animal models demonstrate the potential of Aminex Resins in therapeutic applications in human diseases, such as cancers and inflammatory disorders. Clinical trials of radionuclide-labeled Aminex-based microspheres for the treatment of HCC and mCRC warrant future studies to maximize survival options for patients with aggressive liver malignancies. Further, Bio-Rad’s commitment to quality, customer support (from procurement to process scale-up and improvement), security of supply, and sustainability ensure the feasibility of employing chromatography resins for therapeutic purposes.
Visit our website to request more information and discuss your application with our global teams of scientists, or to request resin samples.
Share your success: email process@bio-rad.com and tell us how Bio-Rad resins have helped you achieve your goals, advance science, and save lives.
References
Bazak R et al. (2014). Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol Clin Oncol 2,904–2,908.
d’Abadie P et al. (2021). Microspheres used in liver radioembolization: From conception to clinical effects. Molecules 26, 3,966.
Emmons EC et al. (2022). Survival and toxicities after 90Y transarterial radioembolization of metastatic colorectal cancer in the RESIN registry. Radiology 305, 228–236.
Häfeli U (2001). Review: Radioactive microspheres for medical applications. In Physics and Chemistry Basis of Biotechnology, M. De Cuyper and J.W.M Bulte, eds. (Dordrecht, Netherlands: Springer), pp. 213–248.
Jernigan SR et al. (2015). Selective internal radiation therapy: Quantifying distal penetration and distribution of resin and glass microspheres in a surrogate arterial model. J Vasc Interv Radiol 26, 897–904.
Kamble Ravindra K KRK et al. (2014). Current trends: Ion exchange resinates in controlled drug delivery. Int J Pharm Drug 2, 1–11.
Kim KK and Pack DW (2006). Microspheres for drug delivery. In BioMEMS and Biomedical Nanotechnology, M. Ferrari et al., eds. (New York, New York: Springer), pp. 19–50.
Natsume A et al. (2000). Cationic liposome conjugation to recombinant adenoviral vector reduces viral antigenicity. Jpn J Cancer Res 91, 363–367.
Sánchez-Trasviña C et al. (2021). Purification of modified therapeutic proteins available on the market: An analysis of chromatography-based strategies. Front Bioeng Biotechnol 9.
Schubiger PA et al. (1991). 90Y-resin particles–animal experiments on pigs with regard to the introduction of superselective embolization therapy. Int J Rad Appl Instrum B 18, 305–311.
Sinha VR et al. (2004). Radioactive microspheres in therapeutics. Pharmazie 59, 419–426.
Sirtex Medical Ltd and Gray BN (2002). Polymer based radionuclide containing particulate material (International Patent No. WO-0234300-A1). World Intellectual Property Organization.
Steel JC et al. (2004). In-vitro evaluation of ion-exchange microspheres for the sustained release of liposomal-adenoviral conjugates. J Control Release 95, 601–611.
Subramanian S et al. (2018). Preparation and preliminary in vivo evaluation of 166 Ho-labeled microspheres for possible use in radioembolic therapy of liver cancer. J Labelled Comp Radiopharm 61, 509–514.
Welling MM et al. (2023). Microspheres as a carrier system for therapeutic embolization procedures: Achievements and advances. J Clin Med. 12, 918.
Worgall S et al. (2000). Free cholesterol enhances adenoviral vector gene transfer and expression in CAR-deficient cells. Mol Ther 1, 39–48.


