Translational research bridges the gap between scientific discoveries and real-world applications, transforming scientific breakthroughs into life-changing therapies. Success in this endeavor hinges on several factors, from collaboration between scientists and clinicians to asking clinically critical questions early during development. A notable example of this is the approval of Zolgensma, a gene therapy for spinal muscular atrophy (SMA), which marked a significant milestone in the field of translational research.
Zolgensma (AVXS-101) (Novartis, AveXis) is an adeno-associated virus (AAV)-delivered gene therapy for treatment of spinal muscular atrophy (SMA). Its approval by the U.S. Food and Drug Administration (FDA) in 2019 represents a clinical landmark for translational research of gene therapies. Overall, this achievement illustrates how deep knowledge of disease biology, well-defined clinical outcomes, and strategic funding can contribute to the development of transformative therapies.
SMA is a rare genetic neurodegenerative disease caused by mutations in the SMN1 gene, leading to progressive muscle weakness and loss of movement control. SMA is a major genetic cause of infant mortality; indeed, early death by the age of two years or lifelong disability occurs in the majority of cases (Farrar et al. 2017).
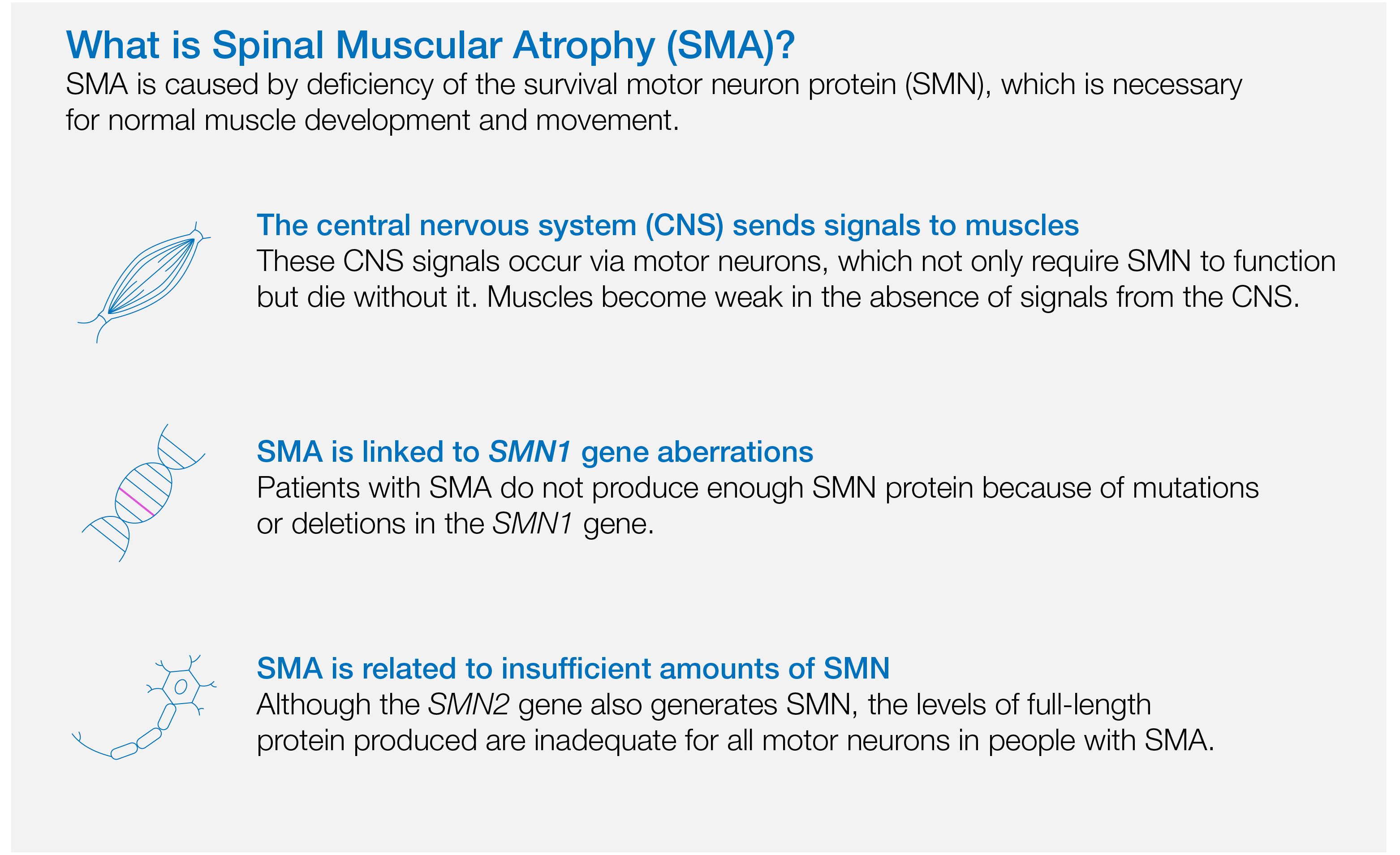
The single-gene nature of SMA rendered it an ideal candidate for gene therapy. As the groundwork for Zolgensma development, a study published in Nature Biotechnology in 2009 demonstrated that AAV type 9 (AAV9) can uniquely deliver genes to the central nervous system (CNS) and that a single intravenous (IV) injection of AAV9 may be sufficient to restore SMN expression in preclinical models (Foust et al. 2009). Building on this foundation, in 2013, AveXis, Inc. received funding through a cooperative agreement program via the National Institute of Neurological Disorders and Stroke (NINDS) supported by the National Institute of Health (NIH) to develop a novel gene therapy to treat SMA (NIH 2013).
Overcoming Challenges in Clinical Development
With this funding, AveXis conducted preclinical development of its lead candidate, AVXS-101, an AAV9-delivered treatment designed to replace a defective SMN1 gene, thus restoring SMN production and protecting motor neurons from cell death. Despite promising initial preclinical results, translating AVXS-101 into a clinical therapy presented unique challenges. For example, SMA is phenotypically diverse, with variable onset and severity (Meyer et al. 2015). To address this diversity, researchers identified and validated sensitive biomarkers such as the compound muscle action potential (CMAP) and motor unit number estimation (MUNE). These biomarkers assisted in predicting patient outcomes in early clinical trials and in demonstrating SMN restoration in mouse models (Arnold et al. 2014).
In 2014, AveXis initiated the phase 1 START trial (ID: NCT02122952) to evaluate AVXS-101’s safety and efficacy following IV delivery. The results of the three-year long study were very encouraging: patients receiving treatment attained motor milestones that had never been achieved in the natural history of the disease, with improved and sustained muscle function. Based on these results, the FDA granted AVXS-101 its Breakthrough Therapy designation in 2016.
Further optimization of AVXS-101 involved delivering the therapy intrathecally, that is, directly to the cerebrospinal fluid; this allowed for lower doses and better motor neuron targeting than single-dose IV injections (Mayer et al. 2015). These findings informed subsequent trials and strengthened the case for FDA approval.
From Clinical Trials to Approval
The phase 3 STR1VE-US trial (ID: NCT03306277), which was launched in 2017, confirmed the safety and efficacy of AVXS-101. Patients with SMA type 1 showed prolonged event-free survival and significant motor function improvement, consistent with the earlier trial results. Recruitment for the European counterpart STR1VE-EU trial (ID: NCT03461289) began in 2018, with outcomes validated at nine different sites across Europe (Mercuri et al. 2021). The promising results garnered industry investment, and AveXis was acquired by Novartis in 2018, which enabled further research and development.
Supported by robust clinical data, AVXS-101 in 2019 received FDA approval under the brand name Zolgensma for use in SMA patients under the age of two years. The European Medicines Agency (EMA) approved it a year later, granting conditional marketing authorization through its PRIority MEdicines (PRIME) program, which accelerates the development of medicines addressing unmet medical needs (similar to the FDA’s Breakthrough Therapy designation).
Refining Treatment Strategies
Zolgensma’s journey continued with the STRONG trial (ID: NCT03381729), in which concerns about IV dosing for older, heavier infants were addressed. Intrathecal delivery led to equivalent CNS distribution at ten times lower doses, improving outcomes while minimizing risks (Finkel et al. 2023). These findings enhanced Zolgensma’s therapeutic reach and will inform future gene therapy development.
A New Horizon for Gene Therapies
The approval of Zolgensma by the FDA in 2019 underscores the transformative potential of translational research for gene therapies. By combining deep understanding of disease pathobiology and developing defined outcome measures, together with funding from cooperative support programs, researchers were able to overcome significant challenges to produce a life-saving therapy. Zolgensma’s development offers a blueprint for advancing future gene therapies, thereby providing hope for individuals affected by rare genetic disorders and reaffirming the promise of translational research.
Visit our website to learn more about translational research and how to successfully navigate the journey from foundational research to manufacturing.
References
Arnold W at al. (2014). Electrophysiological biomarkers in spinal muscular atrophy: Preclinical proof of concept. Ann Clin Transl Neurol 1, 34–44.
Farrar MA at al. (2017). Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol 81, 355–368.
Finkel at al. (2023). Intrathecal onasemnogene abeparvovec for sitting, nonambulatory patients with spinal muscular atrophy: Phase I ascending-dose study (STRONG). J Neuromuscul Dis 10, 389–404.
Foust KD et al. (2009). Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27, 59–65.
Gene replacement therapy clinical trial for participants with spinal muscular atrophy type 1 (STR1VE). ClinicalTrials.gov identifier: NCT03306277. Updated August 16, 2022. Accessed December 23, 2024.
Gene transfer clinical trial for spinal muscular atrophy type 1. ClinicalTrials.gov identifier: NCT02122952. Updated September 15, 2022. Accessed December 23, 2024.
Mercuri E et al. (2021). Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol 20, 832–841.
Meyer K at al. (2015). Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther 23, 477–487.
NIH (2013). Translating a CSF delivered AAV9-SMN for treatment of spinal muscular atrophy.
Single-dose gene replacement therapy clinical trial for participants with spinal muscular atrophy type 1 (STR1VE-EU). ClinicalTrials.gov identifier: NCT03461289. Updated August 16, 2022. Accessed December 23, 2024.
Study of intrathecal administration of onasemnogene abeparvovec-xioi for spinal muscular atrophy (STRONG). ClinicalTrials.gov identifier: NCT03381729. Updated April 24, 2023. Accessed December 23, 2024.


