Bio-Rad is proud to introduce our new real-time PCR (RT-PCR) testing kits for COVID-19. These all-in-one kits are designed for sensitive, specific detection of SARS-CoV-2 alone, or simultaneous detection of SARS-CoV-2, influenza A, and influenza B.
On February 12, 2021, Bio-Rad launched two new RT-PCR kits for COVID-19 testing under an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA).
The Reliance SARS-CoV-2 RT-PCR Assay Kit has primer/probe sets targeting the SARS-CoV-2 nucleocapsid (N) gene (N1 and N2 regions) as well as the human RNAse P (RP) gene as a control.
Designed for the current flu season, the Reliance SARS-CoV-2/Flu A/Flu B RT-PCR Assay Kit detects the SARS-CoV-2 N gene and the human RNAse P gene, plus the M1 gene for influenza A and the NS2 gene for influenza B. The format of the kits is validated on the most popular qPCR instruments and provides many benefits for clinical detection of COVID-19 in Clinical Laboratory Improvement Amendments (CLIA) -authorized laboratories.
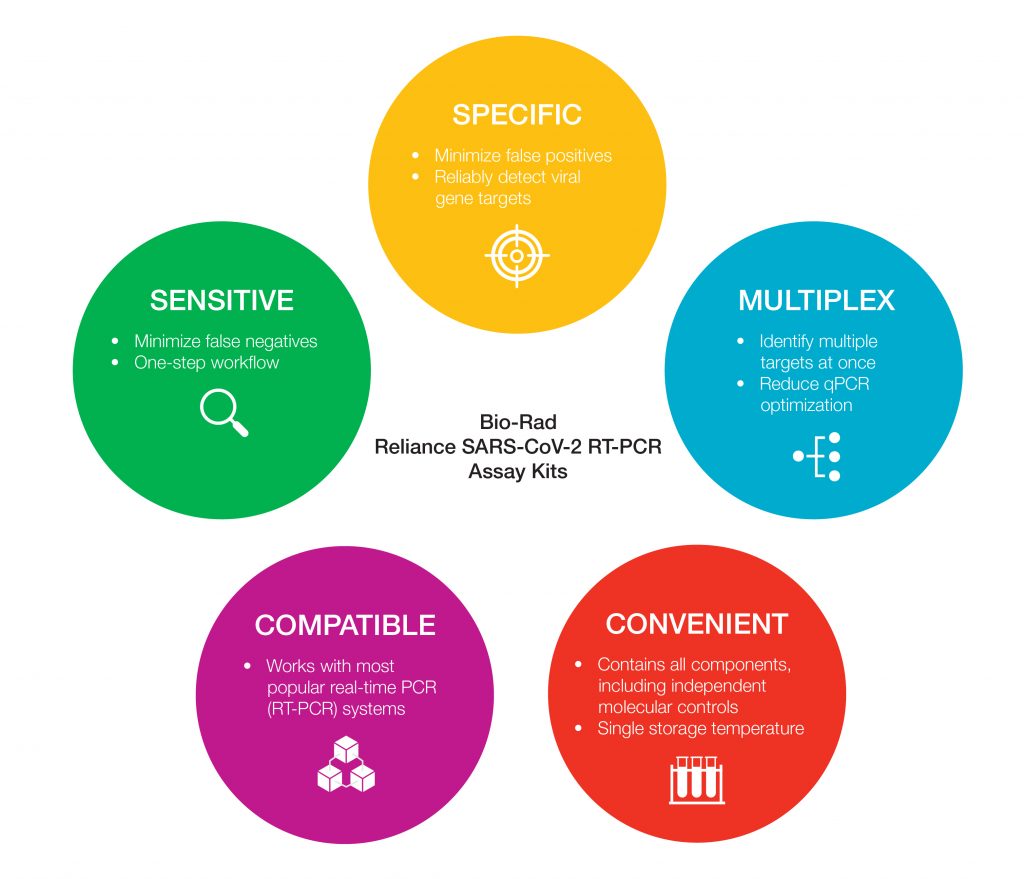
Both kits include all reagents required for 200 reactions:
- Independently developed positive and negative molecular controls from Exact Diagnostics
- Oligonucleotide primers and probes sequences designed by the U.S. Centers for Disease Control and Prevention (CDC)
- Reliance One-Step Multiplex RT-qPCR Supermix optimized for sensitive amplification of multiple targets in a single reaction
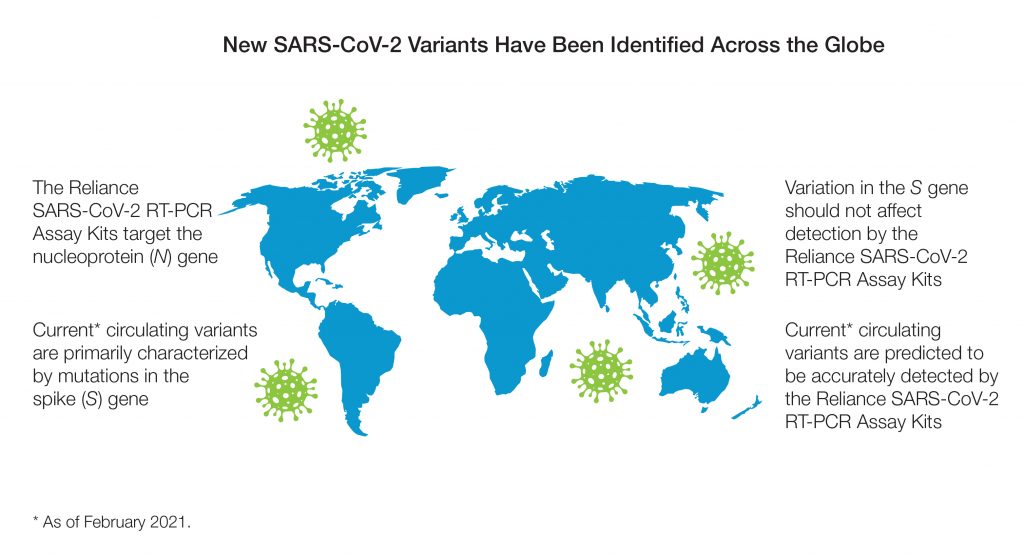
Both kits can be used to detect SARS-CoV-2 nucleic acid in human samples, despite the presence of Spike gene variants. By targeting the nucleocapsid (N) gene, a highly conserved region which is less susceptible to mutation, the predicted inclusivity of the SARS-CoV-2 assay in these kits is not impacted by the current circulating variants. This approach was confirmed by in silico variant analysis against the UK, South Africa, Brazil, and California strains (February 2021).
These new products help support the demand for accurate and efficient COVID-19 clinical testing, as detection is essential to combating the global pandemic.
“Testing that provides greater sensitivity and high throughput are key in helping to reduce the spread of COVID-19,” said Annette Tumolo, Bio-Rad Life Science Group EVP and President. “As the pandemic continues, and with flu season upon us, it is critical for labs to have access to sensitive and reliable tests to help physicians guide patient treatment decisions. We are pleased to receive Emergency Use Authorization for the Reliance SARS-CoV-2 RT-PCR Assay, a test that is suitable for diagnostics labs of any size and throughput, offering clinicians a highly sensitive and robust viral detection workflow.”
Table 1. Features of the Reliance SARS-CoV-2 RT-PCR Assay Kits.
| Features | Reliance SARS-CoV-2 RT-PCR Assay Kit |
Reliance SARS-CoV-2/FluA/FluB RT-PCR Assay Kit |
| Intended use* | Qualitative detection of nucleic acid from SARS-CoV-2 in specimens | Simultaneous, qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B viral nucleic acid in specimens |
| Sensitivity (limit of detection) |
125–500 copies/ml | SARS-CoV-2: 125–250 copies/ml Influenza A: 501–1,002 copies/ml Influenza B: 862 copies/ml |
| Targets detected | SARS-CoV-2 nucleocapsid (N) gene (N1 and N2 regions) Human RNaseP (internal control) |
SARS-CoV-2: nucleocapsid (N) gene Influenza A: matrix (M1) gene Influenza B: nonstructural 2 (NS2) gene Human RNaseP (internal control) |
| Instrument compatibility | Bio-Rad CFX Opus 96 Real-Time PCR System Bio-Rad CFX Opus 384 Real-Time PCR System Bio-Rad CFX96 Touch Real-Time PCR System Bio-Rad CFX384 Touch Real-Time PCR System Bio-Rad CFX96 Dx System Applied Biosystems 7500 Fast Real-Time PCR System |
Bio-Rad CFX Opus 96 Real-Time PCR System Bio-Rad CFX96 Touch Real-Time PCR System Bio-Rad CFX96 Dx System Applied Biosystems 7500 Fast Dx Real-Time PCR Instrument |
| Validated sample extraction methods | Thermo Fisher Scientific MagMAX Viral/Pathogen Nucleic Acid Isolation Kit QIAGEN QIAamp Viral RNA Mini Kit |
QIAGEN QIAamp Viral RNA Mini Kit |
| Throughput | Up to 1,524 samples per day on a 384-well platform | Up to 372 samples per day on a 96-well platform |
* Refer to product instructions for use (IFU) for additional information.
To learn more about these COVID-19 testing solutions, see these supporting documents:
- Reliance SARS-CoV-2 RT-PCR Assay Kits: Press Release
- Reliance SARS-CoV-2 RT-PCR Assay Kits: Product Information Sheet
- Reliance SARS-CoV-2 RT-PCR Assay Kit: Instructions for Use
- Reliance SARS-CoV-2/Flu A/Flu B RT-PCR Assay Kit: Instructions for Use
These products have not been FDA cleared or approved but have been authorized for emergency use by FDA under a EUA for use by authorized laboratories;
Reliance SARS CoV-2 RT-PCR assay has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens;
Reliance SARS CoV-2/Flu A/Flu B RT-PCR assay has been authorized only for the detection and differentiation of nucleic acid from SARS-CoV-2, influenza A, and influenza B and not for any other viruses or pathogens;
The emergency use of these products is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.