When quantifying nucleic acids via real-time or quantitative PCR (qPCR), high accuracy and reproducibility are essential. However, factors such as inconsistent pipetting and instrument design (specifically a lack of thermal or optical uniformity across the reaction plate) can lead to variations in fluorescence measurement.
Inconsistent pipetting, an often unavoidable human error, is self-explanatory. A complication that arises with certain instrument designs is illustrated in Figure 1. Specifically, the optical design of some qPCR systems creates illumination and detection light paths that vary for each well of the thermal cycler block. The variation in light path lengths produces different absolute fluorescence measurements for wells that contain the same concentration of a reporter fluorophore. A well with a shorter light path, typically located in the middle of the block, will have a higher fluorescence reading compared to a well with a longer light path, typically located on the perimeter of the block.
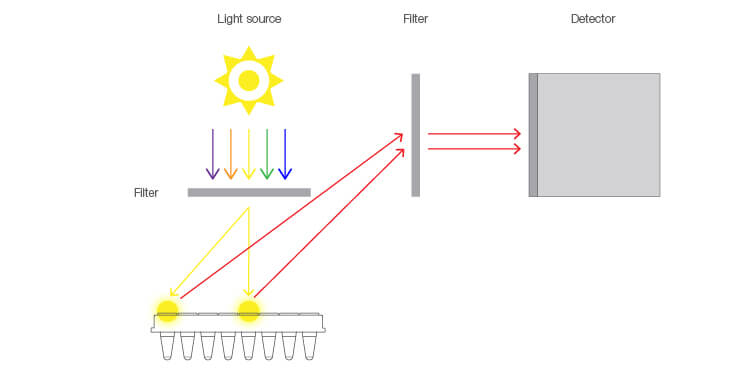
Fig. 1. Light path differences between wells of a lamp-based qPCR system. The well in the center of the block has a shorter light path, which results in a higher fluorescence signal compared to the well on the perimeter, which has a longer light path.
To minimize the effects of such factors, a method to normalize the reaction signal across the reaction plate was implemented very early on. A passive reference dye, which, as the name suggests, does not participate or interfere with PCR amplification, is added to the reaction master mix before its dispensation into reaction wells. The signal from the reference dye is detected in a dedicated channel in real time during thermal cycling along with the target-specific signals that are detected in their corresponding channels. The reference dye signal is used to normalize target-specific signals across the plate to improve well-to-well quantitation reproducibility.
ROX Is the Answer…Or Is It?
By far the most common passive reference dye used for signal normalization is ROX, or 6-carboxy-X-rhodamine. However, depending on the qPCR platform you work with, different concentrations of ROX are needed (Table 1). This need for varying amounts basically has to do with the light source in the particular instrument you are using. In “low ROX” instruments, you get optimal excitation of the ROX dye at ~575 nm and hence a strong ROX signal. In instruments with a “high ROX” requirement, sub-optimal excitation of the ROX dye at ~488 nm leads to a weak ROX signal, which can be overcome by using a high concentration of ROX (Figure 2A). As a result, you either have to spike in your own reference dye or carefully select the appropriate commercial reagent preblended with ROX to be used on your specific system. This can be extremely inconvenient and can lead to invalid results if the incorrect amount of ROX is used.
Table 1. Concentration of ROX needed for the various qPCR instruments.
| ROX Requirement | |||
| Low | High | Not Required | |
| Applied Biosystems (Thermo Fisher) |
7500 7500 Fast ViiA 7 QuantStudio Systems |
7000 7300 7700 7900HT 7900HT Fast StepOne StepOnePlus |
|
| QIAGEN | Rotor-Gene 3000 Rotor-Gene 6000 Rotor-Gene Q |
||
| Roche | LightCycler 480, 96 LightCycler 1.0, 1.5, 2.0 |
||
| Bio-Rad | CFX96 CFX96 Touch CFX96 Touch Deep Well CFX384 CFX384 Touch CFX Connect iQ/iQ5/MyiQ/MyiQ2 MiniOpticon/DNA Engine Opticon I and II |
||
| Stratagene | Mx4000 Mx3000P Mx3005P |
||
| Eppendorf | Mastercycler ep realplex 2 Mastercycler ep realplex 4 |
||
Our Universal Solution
Bio-Rad offers a line of Universal qPCR/RT-qPCR Reagents that utilize a novel, patented* technology that negates the need for different, platform-specific reference dye concentrations. Hence, universal. Specifically, a blend of inert fluorescent dyes — namely the ROX dye and a long Stoke-shift dye — is added to our universal supermixes so that, regardless of platform, only the appropriate reference dye will be excited/detected to generate the appropriate level of reference signal (Figure 2B). When our universal supermix is used on a low-ROX instrument, the ROX dye is excited, resulting in the required strength of signal for proper normalization. When that same universal supermix is used on a high-ROX instrument, the long Stoke-shift dye is excited to generate the required strength of reference signal to enable proper normalization on that platform. Importantly, the inert reference dyes are specifically selected so that they do not generate cross-talk signals that would interfere with normalization or target-specific quantitation.
Think about that for a second. Regardless of the instruments used in your lab or your preference for a particular system, you can use the exact same qPCR reagent. Not only does this eliminate the need to buy or spike in dyes, allow easy reaction setup, and generate less data variability, it also means that experiments and research can be replicated and compared across different systems, labs, and operators.
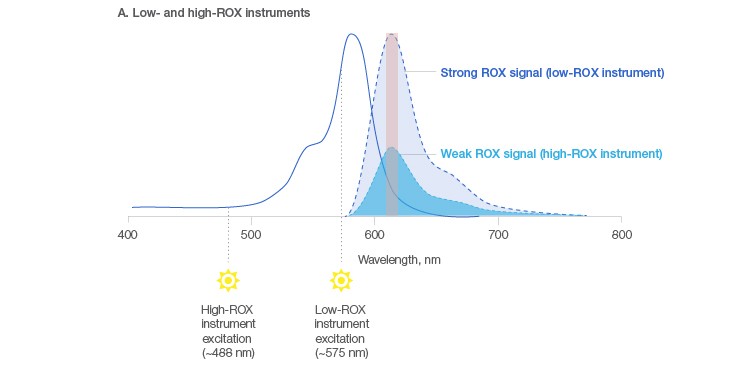
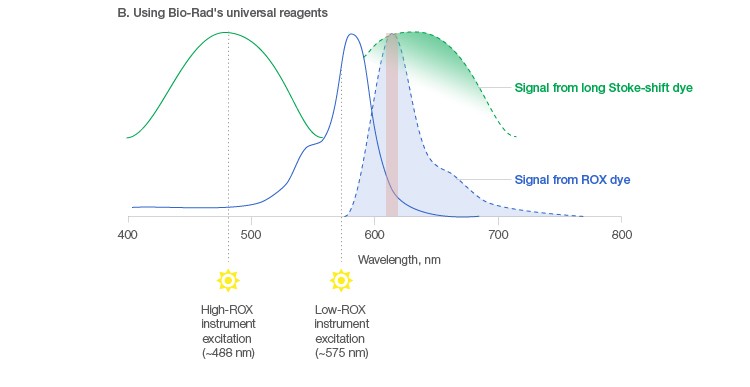
Fig. 2. Passive reference dye signal in low- and high-ROX instruments and using Bio-Rad’s universal reagents. A, optimal excitation of the ROX dye at ~575 nm in low-ROX instruments results in a strong ROX signal, while poor excitation at ~488 nm in high-ROX instruments leads to a weak ROX signal, creating a need for additional ROX when using high-ROX instruments. B, a blend of dyes in Bio-Rad’s universal reagents allows excitation of the ROX dye in low-ROX instruments and the long Stoke-shift dye in high-ROX instruments, negating the need for additional ROX in the latter case. Excitation (—); emission (– – –).
QCing for Lot Consistency
Normalizing signals using reference dyes can help improve assay reproducibility and reduce systematic variations. However, fluctuations in reference dye concentration and consequently in reference signal can negatively impact qPCR data quality. For example, if the reference dye concentration is too low and its signal is detected near the detection limit of the reference channel, it can lead to variation in the normalized target-specific signal and cause variation in quantitation results. Conversely, if the reference dye concentration is too high, it can lead to artificial suppression of the target-specific signal upon normalization and cause delay in Cq value. Recognizing the importance of performance reproducibility in qPCR analyses, we implemented approaches both in product design and manufacturing to ensure the quality and lot-to-lot reproducibility of our universal supermixes.
In a Nutshell
Taken together, it is clear that passive reference dyes are an essential part of supermixes in order to support all researchers with their different qPCR systems. With our universal reagents, any researcher using any qPCR system can use the exact same reagent to obtain optimal quantitation results. Just think about what that can mean for data reproducibility the world over.
See our range of and learn more about our qPCR/RT-qPCR reagents containing universal dyes.
* US 9,493,824
Bio-Rad is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions. All trademarks used herein are the property of their respective owner.

