Choosing the right chromatography resin is a complex and critical decision that can significantly impact the success of your downstream purification workflow, whether for preparative or analytical applications. To make an informed choice, you must consider the scientific principles of the process, practical implications for your specific application, and attributes of the manufacturer. While understanding the types of resins is crucial, selecting the most effective resin goes beyond simply matching a resin type to your target molecule. Several additional factors need to be considered to ensure optimal performance and reliability.

Chromatography resins are available in many varieties and formats.
Chromatography resins are indispensable in the purification process, serving key functions such as capturing, separating, polishing, or analyzing a wide range of biomolecules, including antibodies and small molecules. Resins are also key for in-process tasks like desalting, buffer exchange, and ion removal. Selecting a best-fit chromatography resin is a critical decision with significant implications for the efficiency, yield, and cost-effectiveness of the purification process.
Given the complexity of biomolecular interactions, one or more resin types may be used in sequence depending on the properties of the molecule of interest. For instance, in a typical purification workflow, an ion exchange (IEX) resin, such as a cation exchange (CEX) resin, would be used to capture molecules of interest with an overall positive charge, followed by an anion exchange (AEX) resin under different buffer conditions to capture or adsorb the remaining process impurities. In complex interactions, using more than one resin type, or mode of interaction, as seen with mixed-mode resins (also known as multimodal resins), may maximize purification.
However, the decision-making process involves more than selecting between resin types. The process also involves challenging established practices and considering alternative approaches to improve performance, efficiency, and cost-effectiveness. For example, although Protein A affinity resins are often used as the first chromatographic step for capturing IgG antibodies, alternative workflows may be economically advantageous.
Key Considerations for Resin Selection
There is no one-size-fits-all approach when it comes to resin selection. Whether you aim to enhance purification efficiency, reduce costs, or ensure compliance with industry standards, it’s essential to understand your application objectives, the target’s properties, and your unique workflow conditions. These factors will guide your selection of the most appropriate resin. Here are six key considerations that will empower your decision making and lead to enhanced purification processes:
1
Application objectives on purification strategy
Considering how the purified entity will be used will help to define the purification process objectives and guide resin selection. Key outcomes such as yield, purity, processing time, capacity, and scalability must be carefully balanced according to your application priorities. For example, a resin that provides a higher yield at the expense of purity may not be desirable if product quality is paramount. Similarly, if the goal is to streamline the purification workflow to reduce overall processing time, you may consider trade-offs in purity or yield for faster throughput.
2
Target features and sample impurities
The properties of your target entity, such as size, charge, hydrophobicity, and isoelectric point, and those of the impurities present in the sample will significantly influence resin selection. For example, a protein with an unusually low isoelectric point might be effectively separated from other components in a mixture by using IEX chromatography. This approach takes advantage of charge differences at specific pH levels to achieve the desired separation. Understanding the properties of your target and the impurities present can lead to optimized resin selection and more effective purification.
3
Experimental conditions and workflow
The operational features of your chromatography process, such as flow rate, elution buffer, and buffer components, are also vital in resin selection. A slower flow rate may enhance resolution and separation but could extend the overall process time, making it less suitable for high-throughput operations.
Similarly, the choice between step and gradient elution and the compatibility of the resin with specific pH and salt conditions will depend on the properties of both the target molecule and resin. Buffer screening is also necessary, as the components of the buffer can affect how entities interact with the resin, ultimately influencing the efficiency and outcome of the separation.
4
Resin scalability
One of the most important factors to consider when choosing a resin is its performance at different scales — from laboratory to manufacturing. Some resins that excel in small-scale experiments may behave differently at larger scales due to changes in flow rates, pressure, or binding capacity. For example, size exclusion chromatography may be difficult to scale effectively. Resin cost can also become an issue at larger scales as some resins or associated processes potentially become prohibitively expensive.
Resins may also be available in various formats, including prepacked columns for both lab and manufacturing process-scale applications. Prepacked columns offer notable advantages, such as time and cost savings, reproducibility, and often compliance with good manufacturing practice (GMP) standards. Prepacked columns also eliminate the need for manual packing, thus streamlining the process and minimizing contamination risk.
5
Process economics and sustainability
When evaluating process economics, it’s priceless to consider not only the expense of the resin itself but the total cost, which includes time, labor, and overall process efficiency. For example, a resin that allows for faster flow but produces a lower yield may still be preferred due to reduced personnel time. Nevertheless, achieving both cost-effectiveness and high performance is possible.
A case study in biosimilar purification demonstrated that replacing traditional Protein A capture with a CEX step, followed by polishing with an AEX resin and then a mixed-mode (MM) resin, can reduce reagent costs, increase dynamic binding capacity, and maintain high contaminant-removal levels. This combined IEX:MM approach offers a cost-effective alternative to Protein A purification, improving process economics.
Sustainability is also an important consideration, especially for process-scale applications. For example, Bio-Rad’s CHT Ceramic Hydroxyapatite Media is biodegradable and ships as a dry powder, eliminating the need for prewashing before use — unlike resins shipped in ethanol or benzyl alcohol slurries. Eliminating this step saves water, hazardous waste storage space, and time. These savings can be substantial in process-scale operations, making sustainability a key factor in resin selection.
6
Resin manufacturer considerations
The quality and reliability of the resin manufacturer are just as important as the resin itself, especially in regulated processes like therapeutic production. When evaluating a manufacturer, consider its history, security of supply, capacity to scale up quantities of materials, and ability to distribute globally. Ensure the manufacturer utilizes a quality management system (QMS) to conform to International Organization for Standardization (ISO) standards such as ISO 13485 for the production of medical devices. Furthermore, assess its ability to provide technical and regulatory support, as well as assistance with process development, scale-up, and transfer.
Streamlining Resin Screening Workflows
Given the complexity of resin selection, the value of a thorough screening process before full-scale implementation cannot be understated. Screening involves evaluating various resins to identify the one that best fits the unique characteristics of the target entity and purification process objectives. Screening may even inform an unexpected and advantageous resin selection.
With so many variables at play, a systematic approach such as design of experiments (DOE) offers a robust framework for resin screening. DOE utilizes a three-phase strategy that includes screening, modeling, and optimization. By using specialized software, such as JMP software, DOE enables the simultaneous assessment of multiple variables, accounting for interactions between different factors and revealing how they affect the purification outcome.
Streamlining resin screening workflows is vital for optimizing chromatography processes and achieving optimized purification outcomes. Bio-Rad offers a wide range of resins in prepacked formats to support the entire purification development cycle, from resin screening to small-scale method development and scale-up optimization. These screening tools save process development time and enable high-throughput experiments with minimal sample usage, making them ideal for screening applications, method development, and routine purifications.
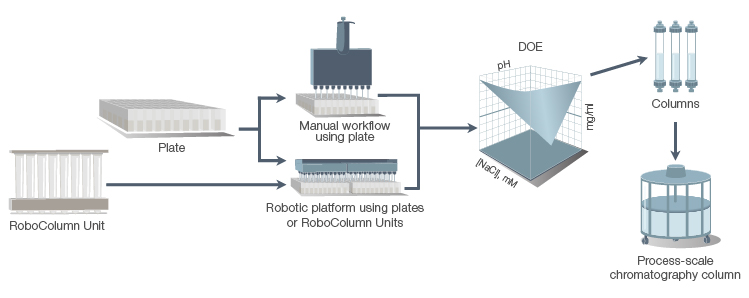
A purification scale-up workflow demonstrates the use of high-throughput screening and design of experiments (DOE) to define an optimal operational window for biomanufacturing.
Which Resin Is Right?
Overall, the choice of resin typically depends on the nature of the entity being processed and the specific application goals. Resins vary widely in composition, structure, and functionality, supporting diverse applications. With so many options available, Bio-Rad provides a range of resources on chromatography, including the new interactive resin selection tool and the updated resin selection guide to aid in resin selection.





