Antibodies are extremely important tools for life science research. It is hard to imagine protein research without antibodies to facilitate immunodetection. However there are significant challenges associated with these crucial reagents, and many published research findings have been reevaluated because of antibodies. For example, a paper in the journal Blood (Elliott S, et al. 2006) called into question the specificity of antibodies claiming to bind human erythropoietin receptors (Epo-R). Casting doubt on these antibodies was alarming, as an entire body of research rested on their validity. This body of research suggested that Epo-R was widely expressed in the cells of a range of tissue types, including tumor cells. If the antibodies were flawed, those published findings might also be mistaken.
Unfortunately, the authors of the Blood article were right. They demonstrated that the Epo-R antibodies exhibited significant off-target binding. Some of the cross-reactivity involved proteins specifically associated with tumor cells. A convincing argument was presented — and has since been supported by other groups (Brown WM, et al. 2007, Kirkeby A, et al. 2007) — that, in many cases, the Epo-R bands “detected” were in fact a heat shock protein (HSP70). One red flag was the molecular weight of the proteins, which ranged from 66 to 78 kDa; Epo-R has an estimated size of 54 to 55 kDa.

Former Scientific Executive Director at Amgen
Steven Elliott, PhD, the first author of the article published in Blood, does not believe the researchers using anti-Epo-R antibodies were ill-intentioned. Still, they failed to use controls, especially negative controls to detect false-positive data, leaving themselves open to confirmation bias (that is, seeing what they wanted to see). As a result, their use of nonspecific antibodies spawned a flood of flawed results, which in turn led to the launch of new clinical trials (Endre Z 2014). Unsurprisingly, no effects were observed in these clinical trials (Endre Z, et al. 2010).
Elliott’s team went on to invalidate several of the most popular Epo-R antibodies in peer-reviewed publications. They also challenged the dogma that Epo-R was widely expressed in many tissue types, including renal cells (Elliott S, et al. 2012) and tumors (Patterson SD, et al. 2015). The problem is: These antibodies remain on the market today and are sold with the same performance claims.
The Magnitude of the Problem
How many more stories like this one are out there waiting to be uncovered? Despite decades of commercial production and distribution, antibodies are still perceived as unreliable. Manufacturing standards and quality control vary wildly between vendors, targets, and even between antibody lots. Consider these sobering statistics:
- Scientists typically have to test between 2 and 4 different products before they find an antibody that works well for their application
- Researchers have said that 50% of their antibodies were of insufficient quality, even though they cite quality as the most important purchasing criteria, according to a research report by 1DegreeBio
- When researchers investigate why their western blots failed, nearly 2 in 3 found that the primary antibody was at fault, according to a Bio-Rad survey
Despite these issues, antibodies remain a critical tool for biology labs; avoiding them is not an option. Fortunately, initiatives are under way to both improve antibody quality and arm researchers with viable tools to routinely assess antibody performance.
In this article we introduce some of these groundbreaking initiatives taken by Bio-Rad Laboratories. But first, it’s useful to understand how we got to this point. Why do many commercial antibodies continue to underperform and fail? What’s keeping scientists from making wiser purchasing decisions? And finally, what can researchers do to improve their own odds of success?
The Pitfalls of Versatility — Why Vendors Can’t Do It All
Competing in a market estimated to be worth $2.0–2.5 billion per year for life science research applications alone, antibody vendors have plenty of incentive to bring a fail-proof product to the market. But after decades of demand, no company has yet been able to do so. Why not?

Senior Lecturer/CiteAb CSO and Co-founder
As a scientist and founder of antibody watchdog website CiteAb, Andrew Chalmers, PhD, has identified versatility as one of the key challenges for vendors.
“People use antibodies for a massive range of applications and also in a range of different species and tissues,” he says. “Companies could never validate an antibody for all of those uses.”
When it comes to manufacturing antibodies for a broad life sciences market, no vendor can validate its products for every possible use. It’s an infinite task: Every lab, every set of protocols, and every group of proteins will be unique. Transportation alone can introduce new variables if temperatures aren’t tightly controlled throughout.
“I haven’t seen a commercial supplier of antibodies that does a full validation that would be acceptable to high-quality venues [facilities]. It really has been the end users’ responsibility to completely, fully validate the antibody,” says Mark Shulewitz, PhD, a senior scientist on Bio-Rad’s content business development team.
Scientists in the Dark
Given the huge scope of applications, all antibodies face performance limits. Some don’t work well when applied to fixed cells; others may bind to a region of the target protein that interferes with its mechanism of action. In most cases, these shortfalls can be managed, provided antibody companies present enough data for buyers to make informed decisions.

Associate Professor of Molecular Microbiology and Immunology
University of Nevada, Reno
Antibody companies don’t always provide sufficient information, though. Or worse, they use improper controls. Unsurprisingly, the information presented is often limited to data that portray the vendors’ antibodies in the best possible light. This denies the researcher the resources required to make an informed decision as to whether a specific antibody will work in his or her assay.
Subhash Verma, PhD, an associate professor of molecular microbiology and immunology at the University of Nevada, Reno, says he has learned to keep an eye out for validation shortcuts that some vendors make. For instance, Verma says some companies rely on overexpressing the target protein to demonstrate performance. However, this does not always translate into performance with real samples.
“An overexpressed target protein study will certainly work, but vendors need to test their antibodies on total-cell lysates,” he says.
Shulewitz notes that on vendor websites, “in most or all cases, vendors tend to show only the positive data, leaving out samples where they don’t see any expression. They also don’t show the entire blot; they crop the blot to show just the region of interest.”
An Incomplete Picture
Scientists look to other sources for information, particularly journal articles that reference an antibody that (presumably) worked in a similar experiment. Citations, however, aren’t enough on their own. Take the current state of western blot data in journals. Only successful western blots are submitted, and negative controls to detect false-positive data are not used. To save space, the blots that are published are often cropped, amplifying the central band. Background noise can be dialed back to produce clearer, less ambiguous results. The aim is typically not to deceive other scientists; but presenting a consistent story is strongly encouraged. Beyond the detrimental impact this practice might have on the reliability of results, it leaves the scientists who read these papers with very little information about the performance of the antibodies used.

Proteomics Researcher at the University of Oslo
Oslo, Norway
Relying too heavily on academic citations creates what Norwegian proteomics researcher Fridtjof Lund-Johansen, M.D., PhD, of the University of Oslo Hospital, calls “survival of the first, not the finest.” This concept captures the feed-forward cycle that occurs as the first antibody to enter a space is used and cited, prompting other researchers to follow suit.
“This becomes a self-perpetuating mechanism,” says Lund-Johansen. “Once an antibody has gotten a certain number of citations, it gets a very strong competitive advantage in the market.”
In recent years, Lund-Johansen has tested more than 6,000 commercially available antibodies, using array technology to work with multiple products in parallel. He says that in his experience, many of the best reagents have no academic citations. Yet with the way the ranking system currently works, any new products that are brought to market — even those of superior quality — are unable to compete with earlier antibodies that are seemingly legitimized by hundreds of citations.
Researchers may be unable or unwilling to do additional studies to determine whether the antibody was properly validated. But if they proceed, they risk publishing additional flawed data, thereby propagating false information that is difficult to correct.
Changing the Status Quo
Bio-Rad is an established supplier of western blot components; however the company’s offerings have lacked a critical component of the workflow: primary antibodies. Having extensively audited the industry by conducting surveys and talking to various thought leaders and scientists, Bio-Rad has come up with the precise solution that the western blotting world needed.
Bio-Rad’s new PrecisionAb™ line of antibodies directly addresses the issues surrounding a lack of data and performance reliability.
- Each antibody is validated on up to 12 whole-cell lysates from distinct cell lines
- Quality control measures are in place to ensure reliability between batches
- Trial sizes allow researchers to test the product in the context of their particular experiment
- A positive control lysate is provided with each antibody for researchers to test the product themselves and compare their data to those on the company website
A large proportion of the improvements have been made possible with specific application focus. Instead of releasing thousands of antibodies to the widest possible market, the line of PrecisionAb Antibodies initially specializes in antibodies that have been well-characterized and stringently screened by western blotting, and includes extensive validation data. If an antibody demonstrates subpar performance — for instance, if it shows a low signal-to-noise ratio or nonspecific binding — it will not be included in the PrecisionAb product line (Figure 1 – FAIL).
A final initiative is in place to add clarity to antibody results: Bio-Rad won’t discard performance data that shows ambiguity in the antibody’s results. The company is publishing the complete testing results for each approved antibody, including results from samples that do not show the target band (Figure 1 – PASS).
Looking at these results, researchers need to keep in mind that a lack of target band in a specific sample “doesn’t mean the protein is not there; it’s just, relatively speaking, much less abundant,” Shulewitz says. “The researchers have an idea of relative expression levels of their target in their sample type, so we’re also guiding that researcher toward samples that work. In addition, we’re providing our own positive control so that customers can verify that the results they’re seeing are just like the ones that we see here at Bio-Rad.”
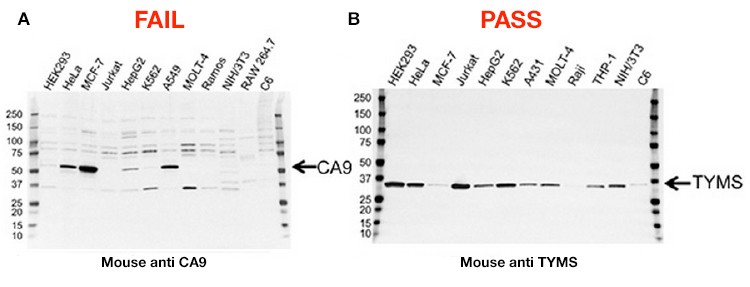
Figure 1. Validation data for two antibodies. A, this carbonic anhydrase IX (CA9) mouse monoclonal antibody failed validation due to nonspecific binding and low signal-to-noise ratio; B, this thymidylate synthase (TYMS) mouse monoclonal antibody passed validation showing high specificity and sensitivity.
This combination of specialization and thorough testing provides the best reliability and value for scientists. Bio-Rad plans to expand its range of antibody products while still ensuring that quality and reliability remain high.
Worldwide tests of PrecisionAb Antibodies, performed in a range of laboratory environments, indicate Bio-Rad may finally restore scientists’ faith in antibody reliability. Beta testers reported that PrecisionAb antibodies typically exhibited greater specificity and sensitivity than the products they normally use. As a beta tester for Bio-Rad’s new PrecisionAb Antibody catalog, Verma reported that all three antibodies he tested worked effectively in low concentrations, which increased the value of the product.
PrecisionAb Antibodies also offer numerous benefits for optimizing protocols and validating antibody specificity in a researcher’s sample, benefits unmatched by other antibody products. These include:
- Trial sizes for all targets — enables researchers to test the antibody and optimize experimental conditions without a large upfront investment
- Complete validation protocol — streamlines optimization and validation, saving time and reagents and delivering top results
- Positive control lysates — provides researchers confidence in their data by simplifying validation of antibody specificity and troubleshooting of western blotting results
To learn more about Bio-Rad’s PrecisionAb Antibodies, visit http://www.bio-rad.com/PrecisionAbs
Check out some tips for choosing the right antibody for your westernblotting experiments.
References
Brown WM, et al. (2007). Erythropoietin receptor expression in non-small cell lung carcinoma: a question of antibody specificity. Stem Cells 25, 714–722. http://www.ncbi.nlm.nih.gov/pubmed/17110616, accessed 7/29-15.
Elliott S, et al. (2006). Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood 107, 3,454. http://www.ncbi.nlm.nih.gov/pubmed/16249375, accessed 7/29/15.
Elliott S, et al. (2012). Lack of expression and function of erythropoietin receptors in the kidney. Nephrol Dial Transplant 27, 2,733–2,745. http://www.ncbi.nlm.nih.gov/pubmed/22167585, accessed 7/29/15.
Endre ZH, et al. (2010). Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int 77, 1,020–1,030. http://www.ncbi.nlm.nih.gov/pubmed/20164823, accessed 7/29/15.
Endre Z (2014). Acute kidney failure study tests early diagnosis and intervention. Press release from the Health Research Council of New Zealand. http://www.hrc.govt.nz/sites/default/files/HRC102%20Endre.pdf, accessed 7/19/15.
Kirkeby A, et al. (2007). Functional and immunochemical characterisation of different antibodies against the erythropoietin receptor. J Neurosci Methods 164, 50–58. http://www.ncbi.nlm.nih.gov/pubmed/17524492, accessed 7/19/15.
Patterson SD, et al. (2015). Functional EpoR pathway utilization is not detected in primary tumor cells isolated from human breast, non-small cell lung, colorectal, and ovarian tumor tissues. PLoS One 10, e0122149. http://www.ncbi.nlm.nih.gov/pubmed/25807104, accessed 7/29/15.

